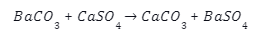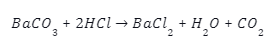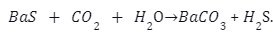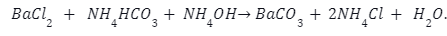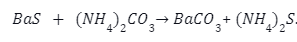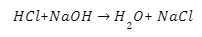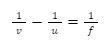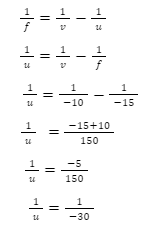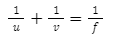Introduction
The chemical symbol for barium chloride is (\(BaC{l_2}\)). It has the appearance of a white salt but is actually an inorganic chemical complex. It gives flames a yellowish-green colour and emits poisonous fumes. It readily absorbs or absorbs water molecules, and may thus cling onto them. As a rule, it dissolves more readily or more easily in water. There is only cautious usage of it in labs and factories due to its toxicity. It has no discernible odour or hue in its pure form.
What is Barium Chloride (\(BaC{l_2}\))?
The empirical formula for barium chloride is \(BaC{l_2}\). The only solvents that work on it are water and methanol, whereas ethanol and ethyl acetate have no effect. To purify the salt water used in caustic chlorine production plants. Sometimes, it is used in the manufacturing of heat treatment salts, which are used in the process of hardening steel.
Its extreme toxicity is shared by the majority of other barium salts that dissolve in water. It changes the colour of a flame to a bright yellow-green. Its molar mass, or molecular weight, is 208.23 g/mol in its anhydrous form and 244.26 g/mol in its dihydrate form (in dehydrated form). About 3.856 g/cm3 is its density. Barium chloride may be dissolved in the methanol, but not the ethanol or the ethyl acetate. When it is dehydrated, it takes on a shape that is similar to an orthogonal structure.
Structure of Barium Chloride \(BaC{l_2}\)
The anhydrous form of barium chloride has a crystal structure that is orthorhombic, while the dehydrated or monoclinic form is extremely unusual. Barium chloride (\(BaC{l_2}\)) is the ionic compound of \(B{a^{2 + }}\) cations and \(2C{l^{-}}\)
anions.
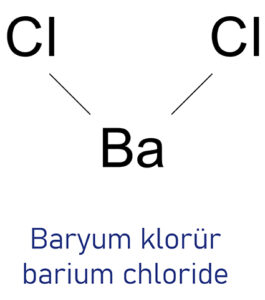
Barium chloride
Preparation of Barium Chloride
Following is the possible methods for the preparation of Barium Chloride (\(BaC{l_2}\))-
Barium sulphate is reacted at high temperature with coke to give barium sulphide.
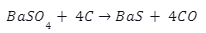
The barium Sulphide is further reacted with dilute hydrochloric acid as follows:
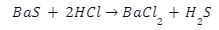
Properties of Barium Chloride \(BaC{l_2}\)
Some of Barium Chloride’s most fundamental characteristics are as follows:
- it takes the form of a white solid, although it can also take the form of a white salt
- Barium chloride has a melting point of about 960 °C.
- It dissolves in water and methanol but not in ethanol or ethyl acetate (EtOAc)
- Barium chloride’s molecular weight is 208.23 g/mol (for the anhydrous form) and 244.26 g/mol (for the dihydrate form) (in dehydrated form).
- The density of barium chloride is approximately 3.856 g/\(c{m^2}\) (in anhydrous form)..
- Barium chloride has a boiling point of roughly 1560 °C.
Uses of Barium Chloride \(BaC{l_2}\)
- For the most part, barium chloride is used in caustic chlorine factories to remove impurities from brine solutions.
- It is employed when carbon steel has to be hardened.
- In addition, it is used in water purification.
- The production of barium chromate, oil lubricants, and similar products all benefit from its presence.
- Purification and the production of salts for thermal processing are two further applications.
- In addition, it is crucial to the creation of some barium salts and a few dyes.
- It may also be used to detect sulphate ions in a sample.
- In the fireworks industry, it’s used to make the crackers a vivid yellow-green colour.
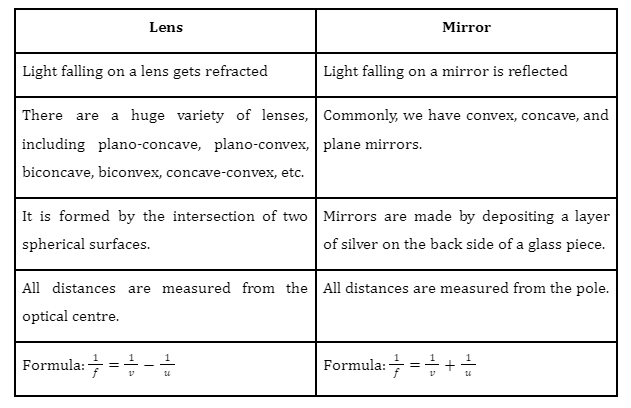
Chemical and Physical Properties
Chemical Properties
When we mix a solution of barium chloride with sodium sulphate, we get a twofold substitution reaction. A white precipitate, consisting of barium sulphate, will appear at the bottom of a test tube after the reaction. The diagram below illustrates the ionic reaction formed by this reaction.
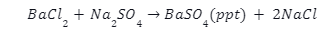
The binary chlorine salt barium chloride readily interacts with water, as is well known. It forms NaCl-like ions when it dissolves in water. It doesn’t have any effect on the solution’s pH because of its neutral behaviour. The following is a response to that:
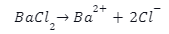
Physical Properties
- As stated in this article, Barium Chloride (\(BaC{l_2}\)) has an orthogonal crystal structure when anhydrous and a monoclinic structure when dihydrate or dehydrated.
- It has a white crystal-like appearance and a molecular weight of 208.23 g/mol (anhydrous) and 244.26 g/mol (hydrous) (in dehydrated form). The anhydrous form has 3.856 g/ml density, whereas the dehydrated form has 3.098. It melts at 963 °C and boils at 1560 °C.
- Barium chloride dissolves well in water and methanol but not in ethanol or ethyl acetate.
Health Hazards Caused due to Barium Chloride
Barium Chloride’s main health risks are:
- Barium chloride’s high toxicity limits its application in industry and labs.
- Barium chloride’s toxicity causes eye, skin, and mucous membrane irritation.
- Inhaling, swallowing, touching, or absorbing it might cause unconsciousness.
- Poisonous barium chloride can damage the kidneys, heart, and brain.
- Aquatic life is at risk.
Summary
Barium chloride (\(BaC{l_2}\)), an inorganic chemical compound, is a white solid or salt. Like barium salts, it is very poisonous. Water and methanol solubility.
It becomes yellow-green towards the flame. We then covered industrial or commercial barium chloride structure and processing. Its many uses include brine or saltwater purification, wastewater treatment, steel case hardening, and more. Finally, we addressed Barium chloride’s physical and chemical features and health risks.
Frequently Asked Questions
1. Is barium harmful to ecosystems?
Very little of these chemicals are preserved in this form after being released into the environment. Insoluble barium compounds have a longer lifetime in the environment and are usually harmless. The two most frequent forms of barium in nature are barium sulphate and barium carbonate, both of which are present in both soil and water.
2. Can barium be inhaled without risk?
A tiny amount of barium sulphate is safe for the lungs to absorb. Barium sulphate can disrupt pulmonary ventilation and perfusion, leading to symptoms including dyspnea, hypoxemia, acute respiratory distress syndrome (ARDS), and mortality if an excessive amount is inhaled or ingested.
3. As an electrolyte, why is \(BaC{l_2}\) so powerful?
\(BaC{l_2}\) is an ionic chemical that dissociates in water to produce ions and conduct electricity. Ionic bonding produces this salt. When electrons are shared between two atoms, an ionic connection forms.
Also Read this article on Barium Carbonate