Introduction
Circuit boards, plastic light fixtures, switches, and sockets can all be made of the synthetic material known as Bakelite. The characteristics include non-absorbency and non-conductivity, as well as high-temperature resistance. Because it is utilized in electronic devices, it is referred to as “Bakelite.” A heated mixture of granular phenolic resin, sawdust, and asbestos is used to make Bakelite, which is then poured into a mould. The phenolic resin was the first artificial resin. Plastic materials come in a wide variety.
Types of plastics
They can be divided into two classes: Thermoplastics and Thermosetting.
Thermoplastics: These are the plastic materials used to make the handles of toothbrushes and bags. Thermoplastics are heated to convert into their different forms.
Thermosetting: After heating, the hardness of this plastic increases. Bakelite is one type of thermosetting plastic.
Bakelite preparation
Bakelite is prepared by following such steps.
- 25 ml glacial acetic acid is taken in a beaker. 12.5 ml 40% formaldehyde solution is mixed with it and heated.
- After some time, 10-gram phenol is added to it and at the end 12-15 ml of HCl solution is mixed.
- It is put in a water bath and heated gently until a solid mass appears.
- Then it is passed through the funnel, fitted with filter paper, and Bakelite remains as a solid compound.
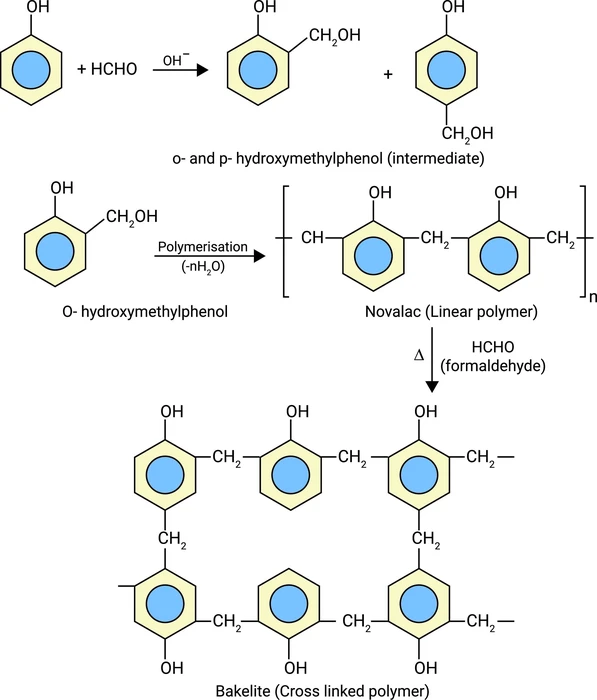
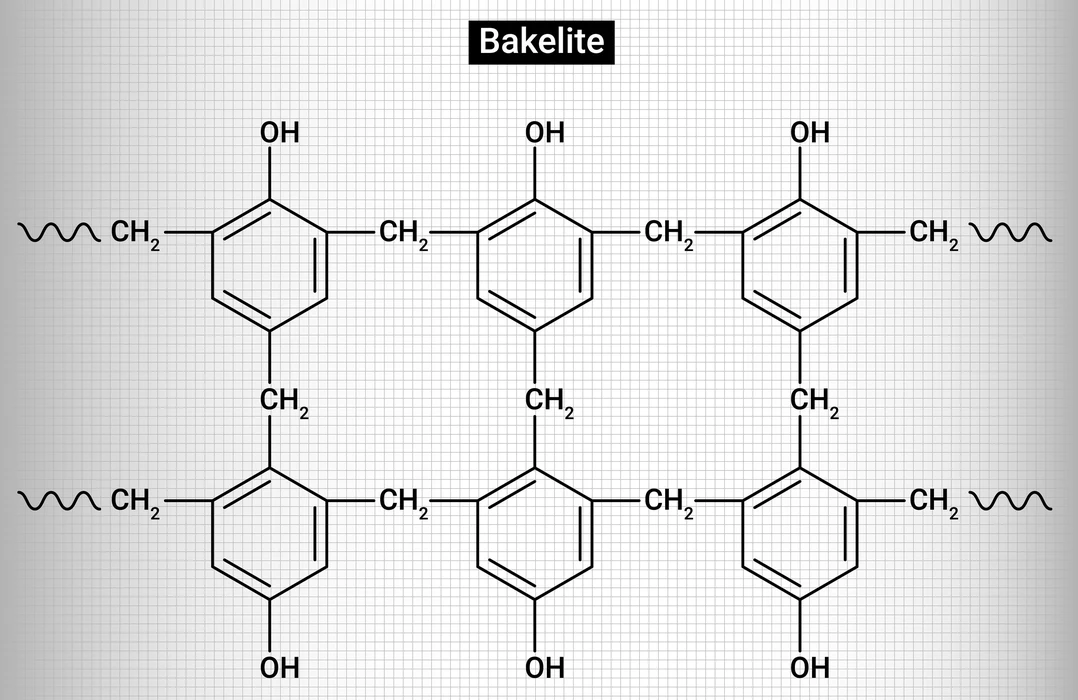
Structure of Bakelite:
In the Bakelite structure, there is a cross-linking between phenol and formaldehyde. Bakelite can be written chemically as\(\;{({C_6}{H_6}O – C{H_2}OH)_n}\).
Properties of Bakelite
- Bakelite can be easily generated, and the mouldings of Bakelite are corrosion and thermal-resistant.
- It would be resistive to current flow because of its low conductivity to electricity.
- Due to its low electrical properties and high heat resistance, Bakelite has also been used primarily in the production of mechanical and electrical components for electrical devices.
- Bakelite contains phenolic components in the structure. For this, it is widely used in bindings.
- Bakelite is moulded at a very rapid rate.
- Bakelite enables the production of extremely smooth mould.
- Bakelite can endure harmful solvents.
- When heated, it melts and solidifies. Then It becomes hard and can be moulded into desired shapes.
- The cost of moulding decreases when an inert filler is used.
Improving your Science concepts. Study Science Lesson for classes 6th, 7th, and 8th.
Uses of Bakelite
- To offer adequate security, it is used in parts that do not use radios or other electronic parts, such as plugs, buttons, hoods, wire cables, brakes, and so on and due to its shaping capacity, it is frequently used in everyday culture.
- Due to its strong tensile strength and thermosetting nature, Bakelite may maintain its shape even after extensive production.
- It has the ability to mould itself in various shapes, making this substance too much useful in modern society.
- It is extensively used in making the parts of washing machines, cooking utensils, watches, toys, and many more.
Importance of Bakelite
- Due to its many important uses as the first synthetic polymer, Bakelite has been appropriately termed “a thousand-use material.” Bakelite is used in the production of many products, including handles of plastic, telephones, ATMs, and so on.
- Due to its strong resistance to both heat and electricity, it is used to produce numerous electronic components, sensors, and vehicle parts.
- Besides these, the features of Bakelite are improved in various ways for better uses.
For these reasons, Bakelite is so important in our daily life.
Summary
Being one of the most widely utilized thermoplastic materials in the nation, it is used to produce many other polymers. It’s vital to remember that because of the distinct characteristics of such a polymer, changing the temperature has little impact on the physical and chemical characteristics of that kind of substance. Having phenolic components in its structure, it is widely used in bindings. But it is very dangerous for human health.
Frequently Asked Questions
1. What occurs when Bakelite is heated?
Ans: As a result of heating a Bakelite, a watery condensation compound (Known as Bakelite A) is produced. Bakelite A becomes dissolved in acetone, alcohol, or extra phenol. Additional heating makes the product somewhat soluble, though the heat can still soften it. The consequence of prolonged heating is the formation of “insoluble, hard gum.”
2. Is Bakelite able to resist fire?
Ans: The handles of many kitchen appliances (fry pans, pressure cookers) are made of sturdy and long-lasting plastic known as Bakelite. It is also a bad conductor of electricity and heat, just like Melamine.
3. Is Bakelite resistant to chemicals?
Bakelite is a tough and chemically inert plastic that was created by combining phenol and formaldehyde. These two substances were obtained from coal tar and wood alcohol (methanol) respectively. Bakelite is inert to chemicals.