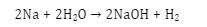Introduction
The whole universe is composed of two parts; system and surroundings. There occurs an exchange of heat between the system and the surroundings. Thermodynamics tells us about the exchange of heat, different forms of energy, and the transformation of energy into work. It also explains some other properties of the system like temperature, pressure, density, enthalpy, entropy, etc.
Define Thermodynamics
Thermodynamics is a topic that derives the relationship between heat, energy, work, and temperature. According to thermodynamics, if the system does the work then its value will be negative and when work is done on the system its value will be positive.
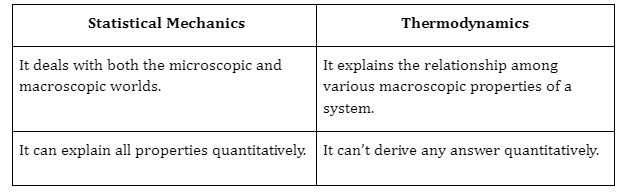
Difference between Thermodynamics and Statistical Mechanics
Define System and Surroundings
System: The part of the universe in which all the matter remains is known as a system.
Surroundings: The other part of the universe outside the system is known as the surroundings. The system and surroundings are divided by a boundary.
Classification of the system:
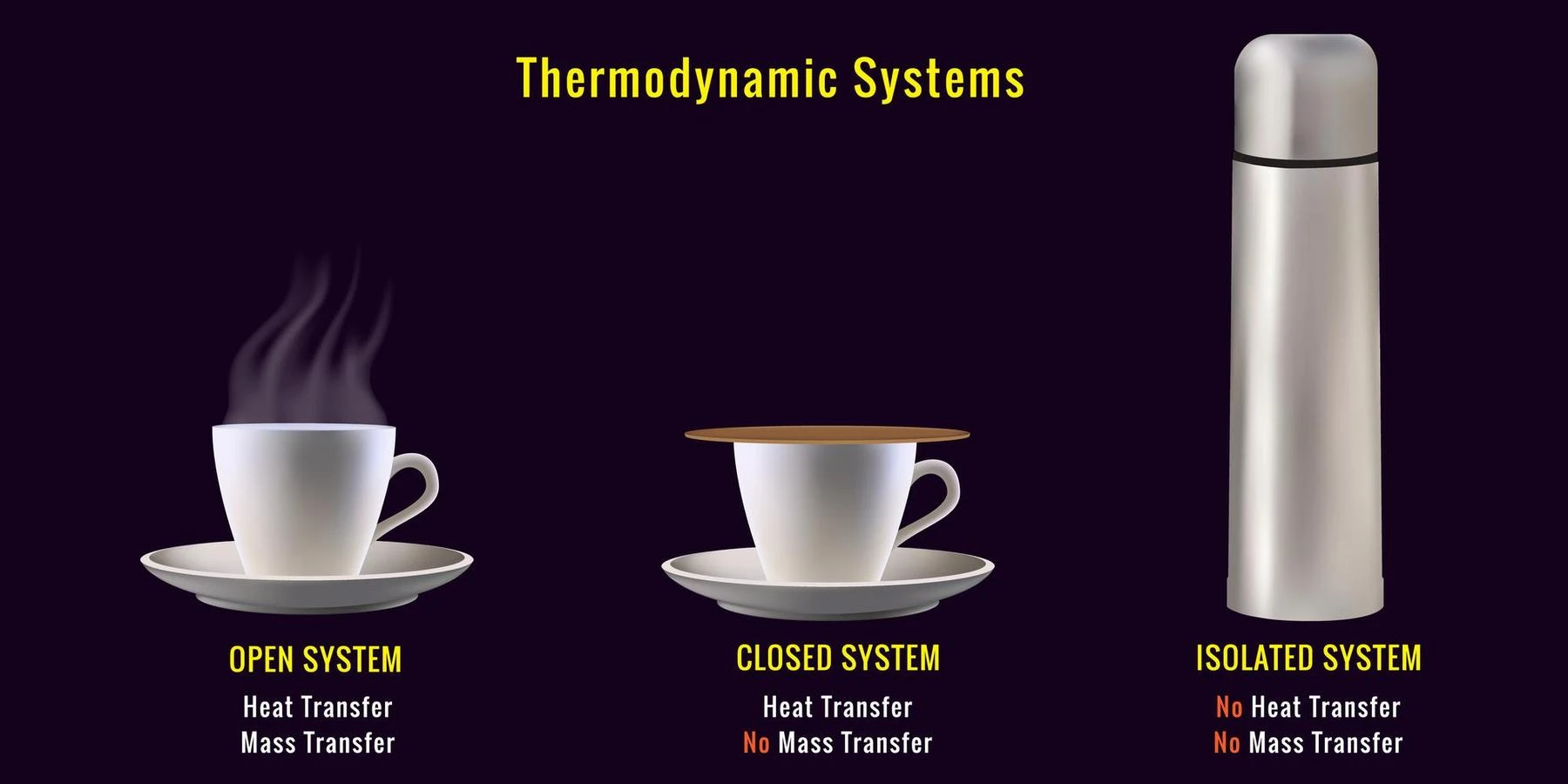
- Open system: It has the capacity to exchange both energy and matter with the surroundings. In an open system, both the temperature T and pressure P are constant. For example, the human body.
- Closed system: This system only exchanges energy with the surroundings. The entropy of a closed system is always constant. For example, water boils using a closed lid.
- Isolated system: It exchanges neither matter nor energy with the surroundings. For example, a thermos flask is an example of an isolated system.
Different types of processes in thermodynamics
- Isothermal process: In this process, the temperature (T) of the system is always constant.
- Isochoric process: Here, the volume (V) of the system is always constant.
- Isobaric process: In this process, the pressure (P) of the system remains constant.
- Adiabatic process: In this process, the change in heat (Q) with the surroundings is zero.
Properties of thermodynamics
- Intensive properties: These properties don’t change with the change in the amount of matter. For example boiling point, melting point, density, etc.
- Extensive properties: These properties highly depend on the amount of matter in the system. For example mass, volume, etc.
Functions in thermodynamics
- State functions: These functions change with the change in the state of a system. For example Enthalpy (H), internal energy (U), entropy(S), and density (d).
- Path functions: Heat (Q) and work (W) don’t depend on the state of a system, but rather depend on the path of a system. They are called path functions.
Define Enthalpy and Entropy
Enthalpy (H): It is a property of thermodynamics that indicates the overall heat capacity of a system. It is expressed by the sum of the system’s internal energy and the product of the pressure and volume of the system.
H = U + PV
Depending on the symbol before the value of enthalpy, any reaction can be classified into two parts.
- Exothermic reaction: The reaction is called exothermic when heat is generated during a reaction. The value of enthalpy in an exothermic reaction is always negative.
- Endothermic reaction: When the system absorbs energy from the surroundings to execute a reaction is called an endothermic reaction. The value of enthalpy in an exothermic reaction is always positive.
Entropy (S): It measures the extent of disorderness of a system. For a spontaneous reaction, entropy S is always negative and for a non-spontaneous reaction, entropy S is always positive.
Thermodynamic potential
Thermodynamic potentials are used to define a particular state of the system. They are internal energy (U), enthalpy (H), Gibbs free energy (G), and Helmholtz free energy (F).
Laws of thermodynamics
- Zeroth law: This law states: “if two bodies A and B are each in thermal equilibrium with some third body C, then they are also in equilibrium with each other.”
- First law: This law states: “Energy can neither be destroyed nor be created, it can only be transferred from one form to another”. It is also called the “Law of conservation of energy.”
ΔQ = ΔU + W
ΔQ= Change in heat of a system.
ΔU = Change in internal energy of a system.
W = Work done
- Second law: This law states: “any spontaneously occurring process will always lead to an escalation in the entropy (S) of the universe.”
\[\Delta {S_{Total}} = {\rm{ }}\Delta {S_{system}} + {\rm{ }}\Delta {S_{surroundings}} > {\rm{ }}0\]
- Third law: This law states: “the entropy of a system approaches a constant value as the temperature approaches absolute zero.”
\[{S_{T = 0}} = 0\]
Daily life examples of thermodynamics
- Human bodies sweat, producing heat from the body.
- Melting of ice cubes.
- Like A thermodynamic system, the human body exchanges mass and energy with the surroundings.
Summary
A type of heat energy that connects with other types of energy is called thermodynamics. Heat or work are two ways that energy is changed or exchanged. In thermodynamics, there are four processes. They are isothermal, adiabatic, isobaric, and isochoric. Thermodynamics explains many important properties of the system. Energy is the dominant focus of thermodynamics which is how it is used and transforms from one state to another. Thermodynamics frequently includes using heat to generate work like in the engines of automobiles and generating work to transfer heat like in the refrigerator.
Frequently Asked Questions
1. Why does thermodynamics emphasize energy?
Ans: The first law of thermodynamics defines that the total energy of the system is always conserved. Neither energy can be created nor destroyed. It is only capable to change from one type to another. Like, in the combustion of fuel the chemical energy is transformed into thermal energy.
2. Why is it referred to as free energy?
Ans: Because it is readily accessible at all times, Gibb’s free energy is known as free energy. If necessary, the reaction can obtain this energy without exerting any effort. Enthalpy (H) and also the product of the system’s temperature (T) and entropy(S) are added to determine the change in Gibbs free energy (G).
G = H +TS
3. What are the drawbacks of thermodynamics?
Ans: Thermodynamics can’t explain any properties of the system quantitatively. It doesn’t include the direction of the flow of heat. It can’t tell anything about the spontaneity of any reaction. These are the drawbacks of thermodynamics.