Introduction
Limestones are a natural source of calcium sulphate. Calcium sulphate is an inorganic compound consisting of \(CaS{O_4}\) and similar hydrates. In the form of anhydrite, it is currently commonly used as a desiccant.
Plaster of Paris is a specific hydrate, and the existence of all the others is attributable to the presence of the mineral gypsum. All of them look like insoluble white particles in water.
It is estimated that the world produces about 127 million metric tonnes of natural gypsum each year. Ca is a metal, but several of its compounds also play essential roles in various sectors and are therefore manufactured on a massive scale.
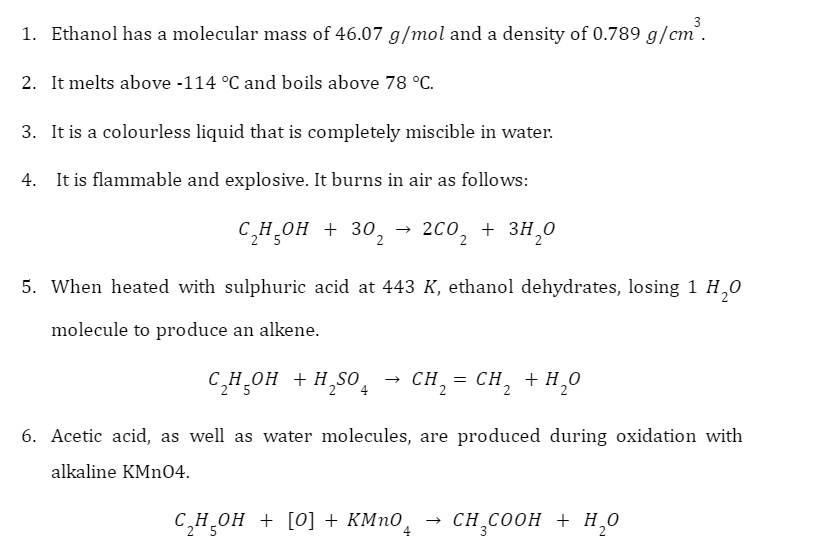
What is Calcium Sulphate?
Calcium sulphate as well as its hydrates are calcium salts. It appears as white particles that are far less soluble in water. The 2 most frequent hydrates include plaster of Paris as well as gypsum. Plaster of Paris seems to be a calcium sulphate hemihydrate produced if gypsum has been heated to 393 K. Whenever heated above 393 K, it produces anhydrite, sometimes termed as “dead burnt plaster,” that reverts to gypsum while added to water. Calcium sulphate is indeed an anti-caking agent, dough developer and strengthener, flour handling agent, pH controller, thickener, as well as yeast food. It has become a fine, odourless and white-yellow powder. It is required in the building sector to make artificial ceilings, plasters, as well as in various other materials.
Structure of Calcium Sulphate
\(CaS{O_4}\) is composed of 1 atom of Ca, 1 atom of S , as well as 4 atoms of O. It is an ionic molecule consisting of 1 calcium cation as well as 1 sulphate anion. The Ca ion has a valency of +2, whereas the sulphate polyatomic ion has a valency of -2. As a byproduct, whenever they interact, the neutral compound \(CaS{O_4}\) is formed.
Hydrous and Anhydrous Forms of Calcium Sulphate
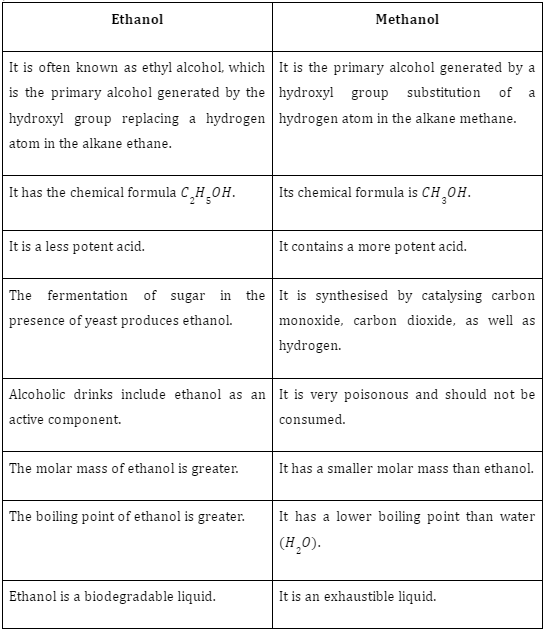
Plaster of Paris and Gypsum
Both gypsum and plaster of Paris appear to be hydrated forms of \(CaS{O_4}\). These two hydrated forms of calcium sulphate are well-known due to their numerous applications in various fields, including medicine and building. Plaster of Paris looks like a white powder that contains gypsum once it’s been hydrated fromCaSO4 salt. C

alcium sulphate hemihydrate is the technical name for them (\(CaS{O_4}.12{H_2}O\)). It’s being used much like a plaster cast to keep shattered bones in place while they heal. Gypsum is heated to 373 K, where it transforms into the compound. Chemically speaking, gypsum would be recognised as calcium sulphate dihydrate. \(CaS{O_4}.2{H_2}O\) must be its molecular formula.
It can be used to cover walls, ceilings, and even decorative pieces for protection and aesthetic purposes. It is impossible to form it into different forms. Once water is added to the plaster of Paris, it becomes gypsum and hardens.

Gypsum Formula
Uses of Calcium Sulphate
- It is most useful in making Plaster of Paris. Because it can be easily transformed into a paste by mixing it with water, \(CaS{O_4}\) powder is particularly useful in this regard.
- It’s a high-quality calcium source.
- It finds widespread use in the building trades and mortar production.
- It is used in instruments for surgery, castings, moulds, and models.
- It can be found in soil conditioners and fertilisers.
- As with alabaster, it can be carved into works of art
- It is being put to use in a process meant to boost the hardness of brewing water.
- It’s used in the production of Portland cement.
- Cosmetics like foot lotions and shampoos include it.
- The dental industry is the primary user.
- Lead and arsenic, both of which contribute to water pollution, can be removed by using calcium sulphate.
- Bread would get its calcium from calcium sulphate, which would also be used to fortify wheat.
Summary
An inorganic form of calcium, calcium sulphate occurs widely in the environment. It is possible to find hydrates of calcium sulphate in the wild. \(CaS{O_4}\) is an inorganic calcium molecule with this chemical formula. Its primary ingredients are the well-known hydrates, Plaster of Paris and gypsum. Water does not dissolve this fine, odourless, white-yellow powder. \(CaS{O_4}\) is used to strengthen flour due to its high calcium concentration. It has many applications in industrial production.
Frequently Asked Questions
1. What is Portland cement?
Portland cement is a binding material that comes in the form of a finely crushed powder, usually grey in colour, and is created by burning and grinding a mixture of limestone and clay or limestone and shale.
2. Is calcium sulphate mined or manufactured?
Commercial calcium sulphate is obtained from naturally occurring gypsum that is extracted or mined.
3. What property of calcium sulphate makes it a good food preservative?
Calcium Sulphate Dihydrate acts as a natural antioxidant, extending the expiry life of food as well as drinks.