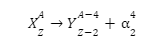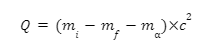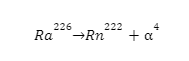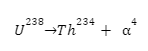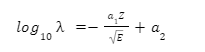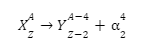Introduction
Radiation refers to the phenomenon when energy in the form of waves or particles is emitted. This can include alpha particles, beta particles, and even gamma photons. Alpha particles are also referred to as alpha radiation or alpha waves, and they consist of two protons and two neutrons.
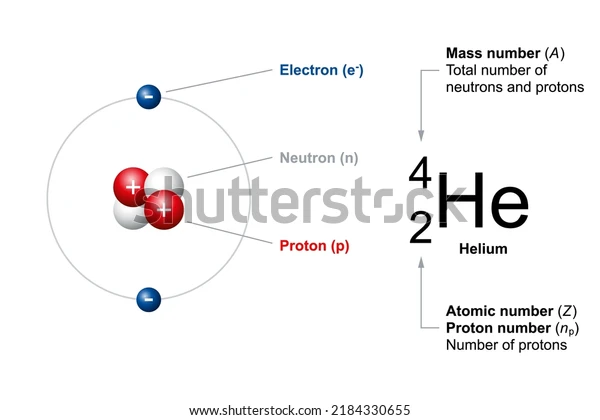
What is an Alpha particle?
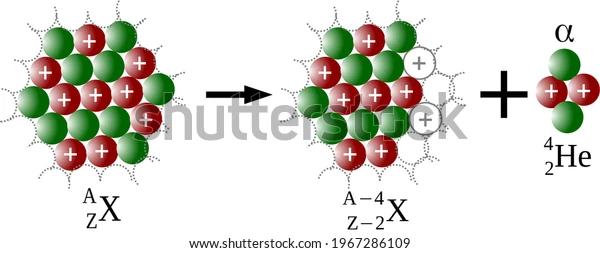
Alpha particles are produced through the α-decay of certain radioactive elements such as uranium, thorium, radium, and plutonium. They are positively charged and are denoted by the symbol . It is interesting to note that these particles are quite similar to helium nuclei and may even be referred to as doubly ionised helium atom \(H{e^{2 + }}\).
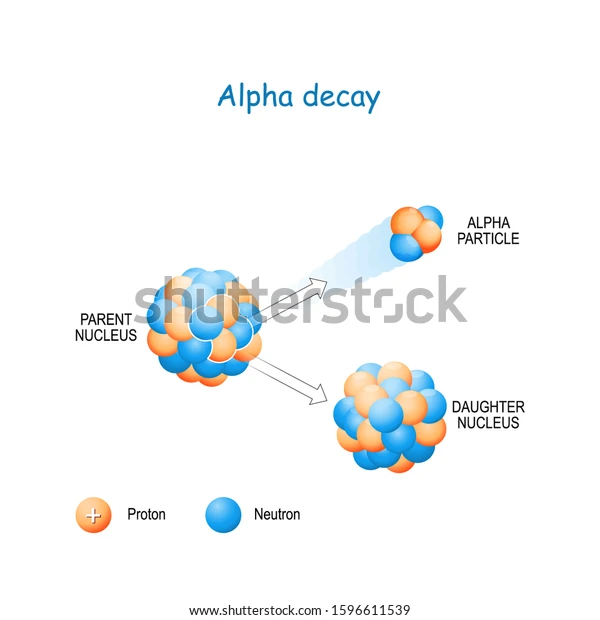
Since these particles carry a double positive charge, they have high ionizing power but do not penetrate too much into matter. When α-decay occurs, the parent element emits an alpha particle, causing it to transform into a different element. For example, uranium-238 undergoes α-decay by emitting α-particles and is transformed into thorium-234.
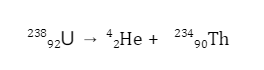
Since the alpha particle carries two protons and two neutrons, the atomic and amss numbers of the parent are reduced by 2 and 4, respectively.
Mass Of An Alpha Particle
The mass of an alpha particle is the same as that of a Helium atom, and is given as:
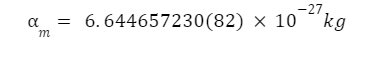
Characteristics of Alpha Particles
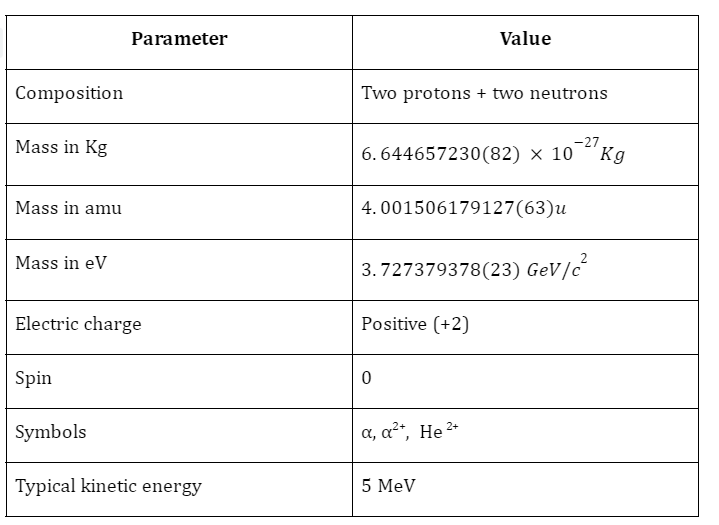
Fundamental Properties of Alpha Particles
- Their velocities can range from \(1.4 \times {10^7}\;to\;1.7 \times {10^7}m{s^{ – 1}}\)
- They tend not to deviate from a straight line path
- They are highly ionising, typically 100 times as ionising as beta particles and \({10^4}\) times as ionising as gamma rays. They can even ionise biomolecules.
- They have low penetration depth and may even be blocked by a sheet of paper.
- Typically, the penetration power is 1/1000 times that of beta rays and 1/100000 times that of gamma rays.
- They can interact with matter via coulomb forces.
- Alpha particles lose kinetic energy quickly and thus, have short range.
- Due to the charge on them, they are easily deflected by electric and magnetic fields. The positive charge makes them attracted to the negative plates.
- They can produce both fluorescence and phosphorescence.
- These particles can be scattered by heavy elements like gold.
- Alpha particles can cause heating effects and though they can affect photographic plates, the effect is very weak.
- Their mass is roughly four times that of Hydrogen atoms.
Uses of Alpha Radiation
- Ra-226 has been used to destroy cancerous cells. Due to their low penetration depth, they only affect the malignant cells and leave normal cells unharmed.
- Americium – 241 is an alpha source that is used in smoke detectors. Smoke ionizes it, which causes an electric current, causing the trigger of an alarm.
- Satellites and spacecraft use Plutonium – 238 in their batteries. The heat produced due to alpha decay is converted into other forms of energy.
- Medical pacemakers used to utilize Plutonium – 238 as fuel source but this practice has non been discontinued.
- Polonium – 21, an alpha emitter, is used to reduce static in industrial settings. Being positively charged, the alpha particles attract the electrons and reduce chances of static electricity.
- Strontium – 90 is an alpha emitter that is used as a fuel source for oceanic buoys by the U.S coast guards.
Summary
Alpha particles consist of two protons and two neutrons bound together, which is equivalent to a helium nucleus. They have high ionization power, allowing them to ionize matter easily.
In nature, alpha particles are emitted from radioactive elements such as U-238 and R-226, and they can also be produced artificially in reactors using radioisotopes like plutonium and californium. These particles have various applications, including cancer treatment, smoke detectors, pacemakers, and thermoelectric generators. The relatively low penetration depth of these particles means that they cannot penetrate the skin and thus, are not harmful.
Frequently Asked Questions
1. How can we detect alpha particles detected?
The following methods can be used to detect charged particles:
- Ionization chamber
- Scintillation counter
- Semiconductor detector
2. How do alpha particles interact with matter?
Alpha particles can interact with matter via coulombic forces. Due to their high ionization power, they can also lose energy by knocking an electron out of the atom they collide with.
3. Discuss the ionization and penetration power of alpha, beta, and gamma rays.
Alpha particles have a high ionization power, which is times greater than beta rays and times greater than gamma rays. However, their relatively higher mass lends them a low penetrating power, which is 1/1000 of beta rays and 1/100000 of gamma rays. But these particles can ionize biomolecules.
4. Is there a difference between alpha decay and alpha particle?
Yes. Alpha decay is the process by which, an unstable nucleus emits an alpha particle and a daughter nucleus. Alpha particles are the particles emitted in this process and are equivalent to doubly positively charged Helium atom.
5. Are alpha particles dangerous? How can we protect ourselves?
Alpha particles have high ionisation power, but their penetration depth is relatively low, which means they cannot penetrate the outer layer of our skin and thus, aren’t that harmful.
However, if you inhale or ingest an alpha source, it can affect your organs internally, which may prove dangerous. The alpha radiation can reach your lungs, which may cause cancer. When consumed through water, it can affect the kidneys. Further, high levels of exposure to alpha radiation can lead to DNA damage, and even larger exposure can cause Acute Radiation Syndrome.
Protection from alpha radiation
Protection from alpha radiation is simple and may be achieved as follows:
- Either decrease time of exposure or,
- Move away from radiation source or,
- Use a shield.