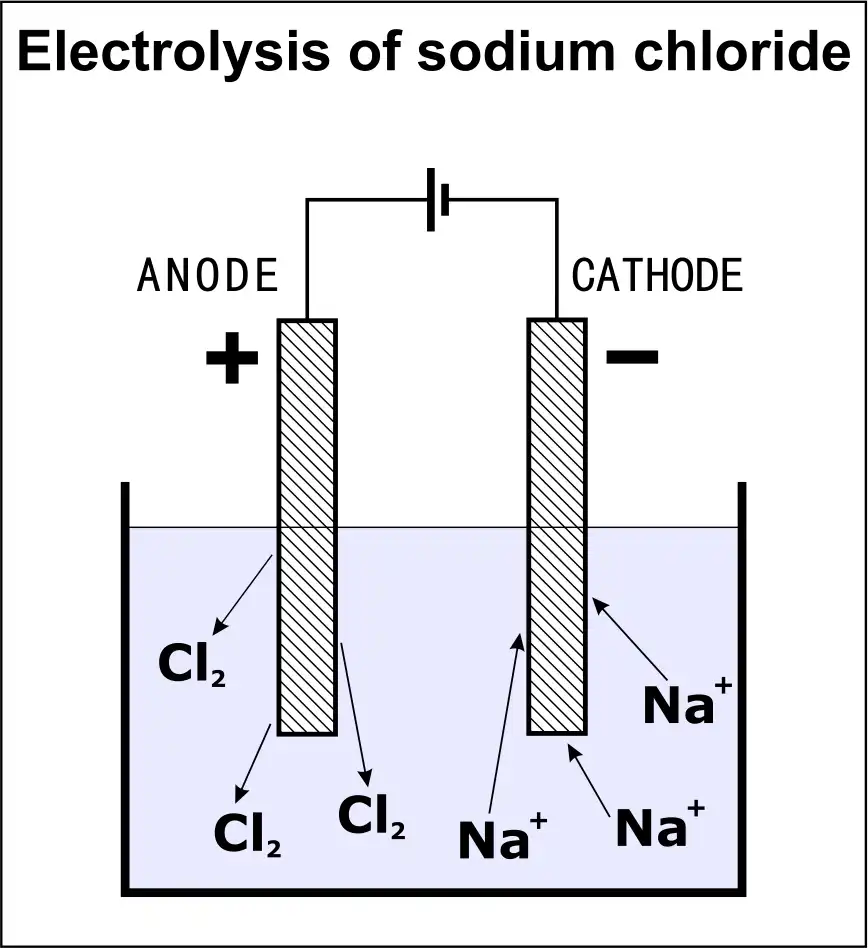
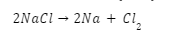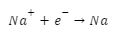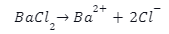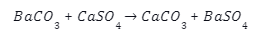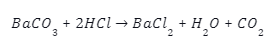Introduction
Poly halogens can be broken down into subgroups. Due to their widespread use, important poly halogens include Freons, DDT, and carbon tetrachloride. Carbon tetrachloride is a colourless, combustible liquid with no discernible odour. Commercial and household use of carbon tet as a cleaning agent was popular before 1970.
Users are put in danger when these refrigerants escape into the air. This calls for the development of innovative, safe, and non-toxic refrigerants. DDT (dichloro-diphenyl-trichloroethane) was developed in the 1940s and was the first of the modern synthetic pesticides. It was first employed to treat military and civilian populations for insect-borne ailments, including malaria and typhus.
Polyhalogen Compounds
Polyhalogen compounds are those that include several halogen atoms (elements in group 17 of the modern periodic table). In both manufacturing and farming, poly-halogen compounds are a common staple. They have several applications and are widely employed as pesticides, solvents, and anaesthetics.
There are several important poly-halogen compounds, but some of the most well-known are methylene chloride, chloroform, carbon tetrachloride, iodoform, DDT, and benzene hexachloride.
Freons
Freons, or chlorofluorocarbons, are a popular refrigerant. Fluorine and chlorine atoms are substituted for the hydrogen atoms in methane (\(C{H_4}\)) to produce it. The properties of CFCs may be altered by including different numbers of chlorine and fluorine atoms. The rule of 90 is used as a naming convention for chlorofluorocarbons. The CFC is commonly referred to as CFC-n, where n is any of the numbers listed below. Following that pattern, we may deduce n by subtracting 90 from the total number of fluorine, hydrogen, and carbon atoms given. If, for example, a CFC’s formula is \(CC{l_3}F\), then that CFC is designated as CFC-11.
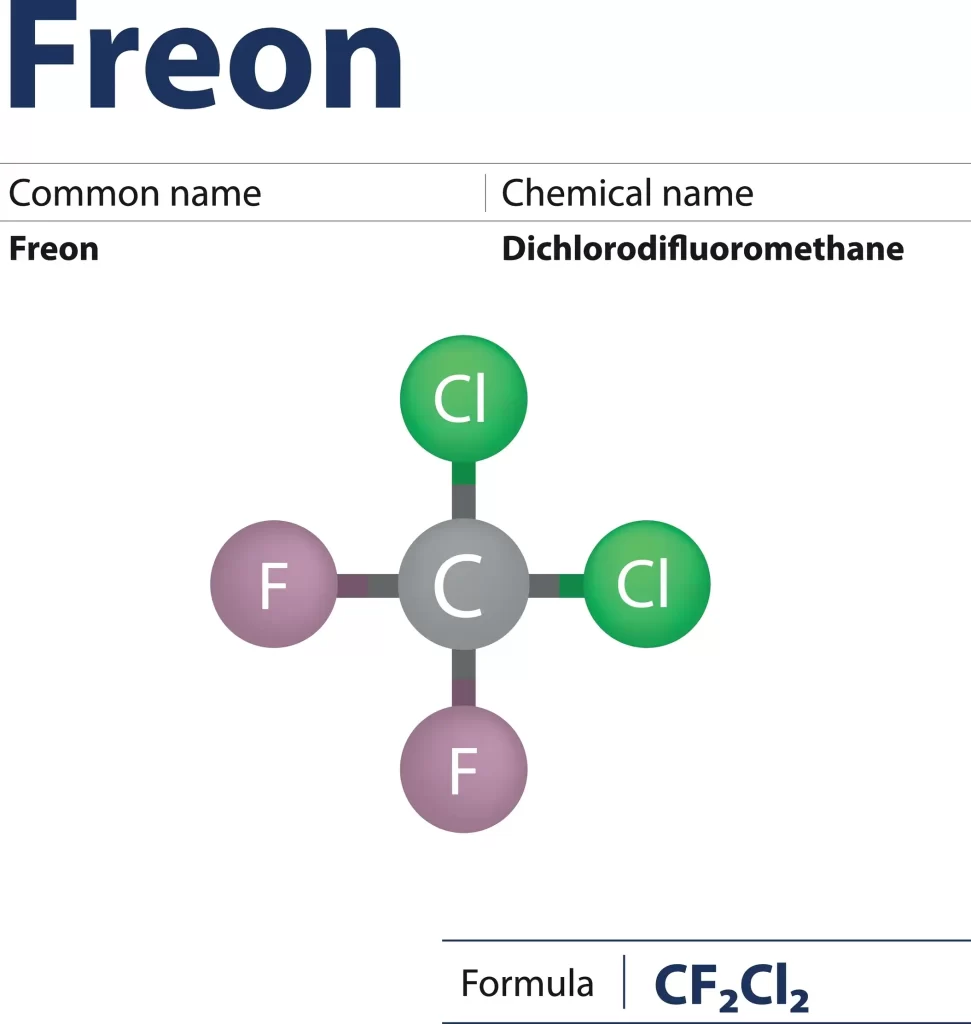
Freon Structure
Structure
Molten sodium and heated, concentrated mineral acids have no effect on Freons. Therefore, as the ratio of fluorine to carbon atoms in Freon gas increases, the length of the resulting solid C-F bonds decreases. C-F bond lengths range from 1.29 to 1.358 angstroms for molecules like \(C{H_3}F\), \(C{F_2}\) etc.
Synthesis
Antimony fluoride reacts with carbon tetrachloride to produce freon and Antimony Chloride, which acts as an autocatalyst.
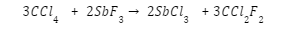
To create chlorofluorocarbons, chlorinated methanes and ethanes are commonly subjected to a halogen exchange reaction. The process of converting chloroform into chlorodifluoromethane is outlined below..
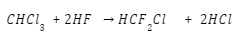
Uses
Due to their low boiling points and low viscosity, freons are widely used as refrigerants in:
- Mechanical cooling and refrigeration devices
- Aerosol propellers
- Ingredients for Foam Blowing
- Solvents
- Glass and intermediate polymer coolers
- Inhalants are widely used legal medications that are ingested through the respiratory system. Inhaling gasoline, paint thinners, sprays, or refrigerant gases is a common way to get high.
DDT
The chemical formula for DDT, or dichlorodiphenyltrichloroethane, is \({C_{14}}{H_9}C{l_5}\). This chemical compound is a crystalline solid that is odourless, tasteless, and colourless under typical pressure and temperature conditions.
In 1939, Swiss chemist Paul Hermann Müller developed DDT’s insecticidal properties. In the latter years of World War II, DDT protected civilians and military personnel from insect-borne diseases, including malaria and typhus.
Structure
DDT is composed of 2 phenyl groups with five carbons as substitutions. The chemical formula of DDT is \({C_{14}}{H_9}C{l_5}\). DDT and its IUPAC name is 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane.
Synthesis
To produce DDT, a mixture of chloral and chlorobenzene (in a ratio of 1:2) is cooked in strong sulfuric acid.
Uses
In addition to solutions in xylene or petroleum distillate, DDT is also available as emulsifiable concentrates, water-wettable powders, granules, aerosols, smoke candles, vaporisers, and lotion charges. Agriculture made heavy use of DDT between the years 1950 and 1980. Fifteen different firms around the United States worked together to produce it. DDT was also used inside structures as a pesticide. Malaria, typhus, body lice, and the bubonic plague were some diseases it was used to combat.
Carbon Tetrachloride
The chemical tetra chlorocarbon was created in a lab; it does not occur naturally. It’s a transparent liquid with a barely discernible sugary scent. Benziform, perchloromethane, methane tetrachloride, carbon chloride, and methane tetrachloride are some of its alternate names. Carbon tetrachloride is a colourless gas that regularly floats in the air.
Structure
The Lewis structure of carbon tetrachloride consists of a single carbon atom in the middle, surrounded by four chlorine atoms. Molecular \(CC{l_4}\). and its electron geometry are both tetrahedral in form. A bond angle of 109.5 degrees is found in \(CC{l_4}\).
Synthesis
French chemist Henri Victor Regnault figured a combination of chloroform and chlorine to produce carbon tetrachloride:
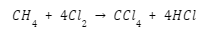
Uses
The various uses of Carbon tetrachloride are:
- Tetrachlorocarbon is used as a solvent in pesticides.
- Fire extinguisher and degreaser
- Remover of Spots
- Making propellants for aerosol cans and cooling fluid.
- As a result of the harm they cause, only a small number of industrial uses are allowed.
Summary
Carbon compounds with more than one halogen atom are referred to as poly halogens. Polyhalogen compounds include freons, DDT, and carbon tetrachloride, amongst others. These poly halogens can be utilised in a variety of contexts. While Freons are used to produce refrigerants and aerosols, DDT and carbon tetrachloride are utilized in the agriculture sector as effective insecticides.
Frequently Asked Questions
1. What effects are associated with the exposure of Freons to UV light?
In the event that Freon does in fact include atoms of chlorine, the chlorine that is extracted from Freon by UV light forms a chemical connection with ozone.
Because of this, ozone cannot quickly revert back to its natural state, which leads to the destruction of ozone and the formation of ozone holes in space.
2. How is DDT toxic?
DDT is toxic because it causes severe diseases in human and animal bodies when ingested. It can cause growth defects, and reproductive issues, it is carcinogenic and it can also cause nervous diseases.
3.What environmental act talks about carbon tetrachloride?
Carbon tetrachloride was a chemical that was used for dry cleaning and as a fire extinguisher before it was made illegal worldwide in 1987 under the Montreal Protocol. It damages the ozone layer and contributes to the ozone hole that has formed over Antarctica.