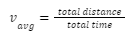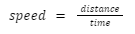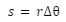Introduction
Astrophysics deals with the knowledge of the universe beyond this Earth. It explores the processes that occur in the depths of space and astrophysicists also study the physics of the various planets and stars in and beyond and the solar system.
Closer to home, we have a field of science known as geology, which is the study of the Earth’s physical structure and substance. Our knowledge of what lies inside the Earth as well as various phenomena that occur due to changes within this composition is all classified under geology. In this article, we are going to explore what the Earth is made out of and how its composition is classified.
What is Earth?
Earth is the third planet in our solar system and the fifth largest by size. It is a terrestrial planet, meaning that it is mostly made up of solid, rocky materials. The surface of Earth isn’t smooth. Rather, it is covered in mountains and valleys in some portions while water covers the remaining 70 percent of its surface, earning it the nickname “ocean planet” and giving it the ability to support life. Its atmosphere is mostly composed of nitrogen and oxygen and a strong magnetic field around the planet prevents it from the effects of solar radiation. Each day on Earth lasts 24 hours and each year comprises 365.25 days. Interestingly, Earth is the only planet in the solar system that has exactly one natural satellite: the moon.
Earth’s Magnetic Field
The intrinsic, natural magnetic field of the Earth arises due to the convection currents developed inside the core of the Earth and extends all the way into space. It gives rise to a layer of the atmosphere known as magnetosphere and is also known as geomagnetic field.
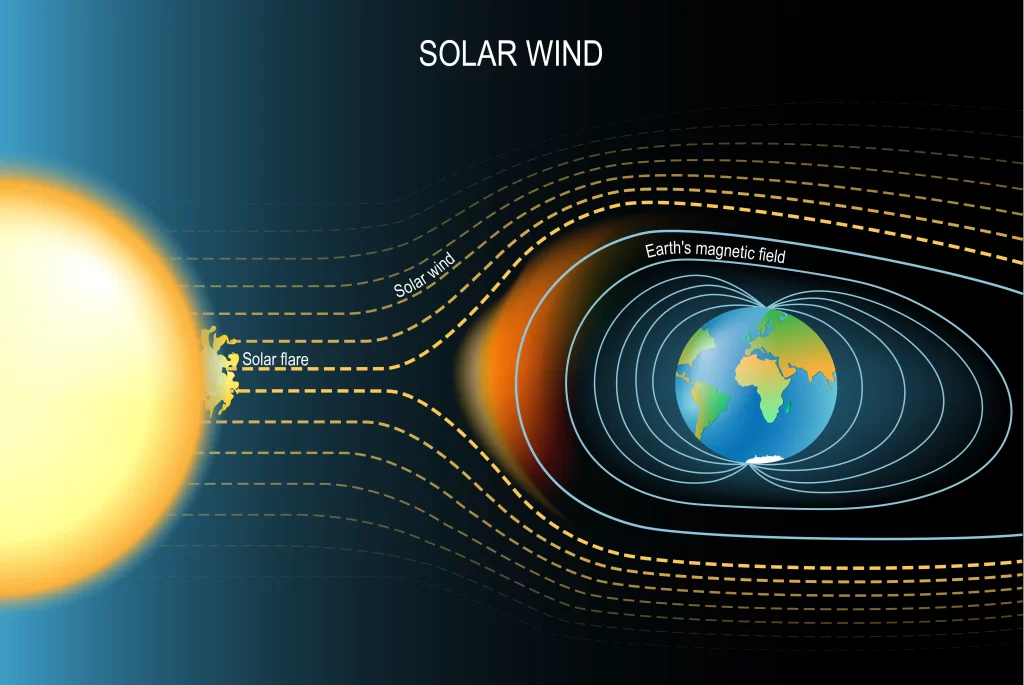
Earth’s magnetic field.
Convection currents in the core develop due to the flow of molten materials like iron inside the core of the Earth. Since these materials contain charged particles, their motion gives rise to a current which creates the magnetic field of the Earth. Due to this field, the Earth can also be thought of as a bar magnet, though its poles do not coincide with the geographical poles.
Layers of Earth
The physical composition of Earth is classified into the crust, the mantle, the outer core, and the inner core. The following figure depicts this classification and we will discuss each layer in detail.
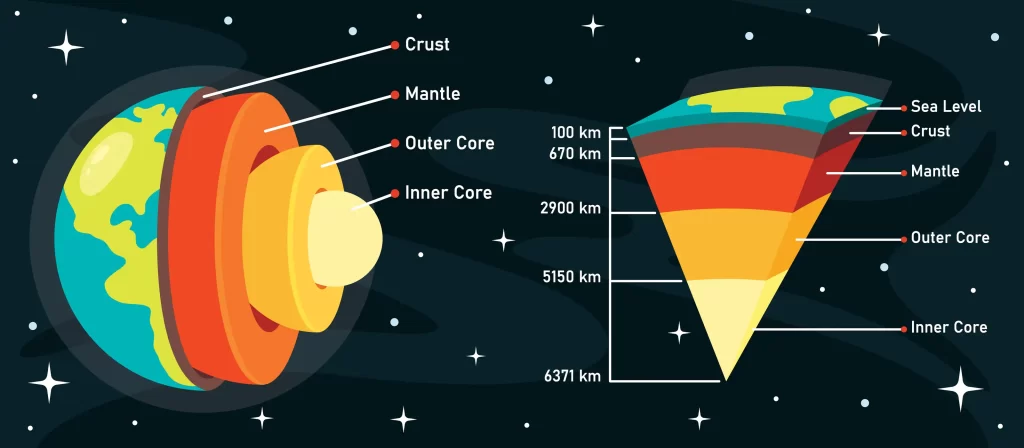
Layers of the Earth
Inner core
- The core is the deepest and hottest layer of the Earth and it lies at the very center.
- It is composed of solid metals like iron, nickel and trace amounts of gold, cobalt, and platinum.
- The inner core has a temperature of around 5,200 ℃.
- Due to the enormous pressure, the core remains solid despite the temperature far exceeding its contents’ melting points.
- The inner core is approximately 1220 km in radius.
- The lighter elements are present near the top of the core while the heavier elements tend to shift downwards. This process is an exothermic one and it heats up the planet.
Outer core
- The outer core lies above the inner core and is the only layer containing iron, nickel, and other substances in a liquid state.
- Its temperature is around 4000-9000 ℉.
- The rotation of the Earth creates a convection current in this layer, forming a magnetic field.
- The outer core is about 2200 km thick and carries 15% of the Earth’s volume.
- Its density is approximately 9.5 to 14.5 g/cc.
- The point of discontinuity between the core and mantle is known as Guttenberg’s discontinuity while the inner and outer cores are separated by Lehmann discontinuity.
Mantle
- The mantle is the solid layer beyond the core and is about 2900 km thick.
- It occupies 84% of the Earth’s volume and is made of elements like silicon and magnesium.
- Its density is in the 3 to 5 g/cc range. The mantle is in a solid state.
- Its upper and lower portions are separated by the Repitti discontinuity.
Crust
- The crust is the outermost, solid layer of the Earth upon which we live. Its thickness varies greatly from 5 to 40 km.
- Most of the crust is covered in water and in oceanic regions, the crust is only 5-10 km thick.
- The crust occupies a meager 1% of Earth’s total volume and it forms the lithosphere of the Earth.
- The outer crust contains sedimentary rocks made up of silicon and aluminum and has a density of around 3 g/cc.
Summary
So far, Earth is the only known planet that supports life. It lies third in our solar system and carries its own magnetic field to prevent it from solar winds. The study of the physical structure and substance of Earth is known as geology and in this article, we explored the various layers that make up this planet. These included the crust, the mantle, and the core.
Frequently asked questions
1. What is hydrostatic equilibrium?
Hydrostatic equilibrium refers to the balance between the gravitational force and the pressure of a gas or liquid. It is a self-regulating force.
2. What is the percentage of fresh water on the Earth?
While about 70% of the Earth’s surface is covered in water, only a small 3% of it is fresh and out of this 3%, 1% is in the form of icecaps and glaciers. The remaining 2% is what we use for our daily activities.
3. What are the conditions necessary for the geodynamo process?
The Earth can create its own magnetic field given that:
- It is rotating at a sufficient velocity.
- Its core contains conductors in a liquid state.
- There is enough internal energy to create the convection current.
4. What elements are present in the inner core?
The inner core is mainly made up of iron (around 80%). About 5 to 15% of it is nickel, 2 to 3% is siderophiles, and the remaining 5 to 10% is sulfur and oxygen.
5. Why is the core so incredibly hot?
The heat provided during the formation of the Earth, along with the heat generated due to friction and radioactive decay of elements makes the Earth’s core immensely hot.