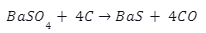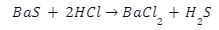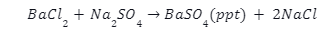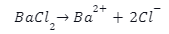Introduction
An immune system is a very essential system of the human body that helps protect one’s self against various harmful materials. The immune system not only fights the antigen upon invasion but also identifies the invader and retains its memory. An allergy is when a person’s immune system reacts adversely to harmless substances such as dust, pollen, any specific edible item, etc. Autoimmune disease on the other hand is a condition wherein a person’s immune system unintentionally targets his/her own cells and tissues.
Allergy
Allergy is the body’s response to common, completely harmless environmental elements that pose no threat to the majority of people. Allergens are the chemicals that cause allergies. People constantly come into contact with the environment, which exposes them to allergens such pollen, dust mites, animal fur, mildew, and insect venom.
While many people lead normal lives, some people experience negative side effects after being exposed to certain substances.
Allergic responses can be-
- A minor allergic reaction- It may only affect one area of the body that has been exposed to an allergen.
- A moderate allergic reaction-If the allergen spreads and affects nearby body parts then it is a moderate allergic reaction.
- A severe allergic reaction- If a sudden, life-threatening ailment develops, then it is a severe allergic reaction.
Symptoms of allergy
Based on the allergen and the site of exposure, an allergy can result in a variety of reactions, some of which are described below as frequent ones.
- Red and inflamed eyes, itching
- Sniffling and a runny nose
- Hives or skin rashes
- Swelling of the lips and mouth
- Itchiness in the mouth,
- Persistent cough
- Inability to breathe
- Chest constriction,
- Vomiting and nauseous
- Diarrhea
- Headaches
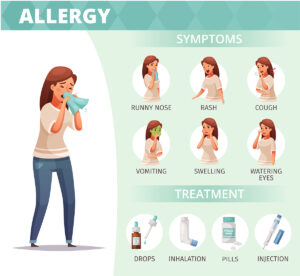
Symptoms of allergies
Autoimmunity
Autoimmunity is the immune system’s reaction against oneself and healthy cells, which causes organ failure or damage along with significant physiological changes. The immune system of the human body triggers an immunological reaction to any foreign substances (antigens) that may be capable of causing disease. White blood cells fight the antigen directly or indirectly create antibodies against it and protect the body.
In autoimmunity the individuals make autoantibodies which attract the autoantigens or healthy tissues and organs of the individual’s body and lead to various kinds of autoimmune diseases. Although the specific causes of some autoimmune diseases are unknown, genetic and environmental factors are being researched as potential contributors.
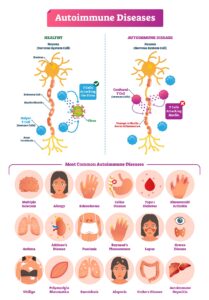
Types of autoimmune diseases
Based on the areas where the autoimmune attack occurs, two types of autoimmune diseases exist they are-
Organ-specific autoimmune disorders:
- As the name implies, the immune system only targets a particular organ or tissue and autoantibodies are focused on that particular organ.
- Examples include Grave’s disease and Hashimoto’s disease, both of which affect the thyroid gland and cause it to malfunction.
- White patches of skin are a symptom of the autoimmune disease vitiligo, which affects just the skin since the melanocytes that produce colour are the target cells for the autoantibodies.
- Addison’s disease develops when the adrenal cortex is attacked by self immune system.
Systemic autoimmune disorders:
- The impacts of systemic autoimmune diseases are extensive, causing many tissue damages in numerous organs.
- Examples include Systemic lupus erythematosus, which causes inflammation, tissue damage, and tiredness in the joints, kidneys, brain, lungs, skin, and blood vessels
- The damage to the articular cartilage that lines the elbows, shoulders, knees, and hips is known as rheumatoid arthritis and is one of example of autoimmune disease
Symptoms of autoimmune diseases
The symptoms of autoimmune disease vary depending on the organ affected.It affects the organ’s physiology and functionality both. The following is a list of the common signs of autoimmunity.
- Fatigue
- Muscle aches
- Aching and swollen joints
- Recurrent mild fever
- Skin irritation
- Legs and hands are numb
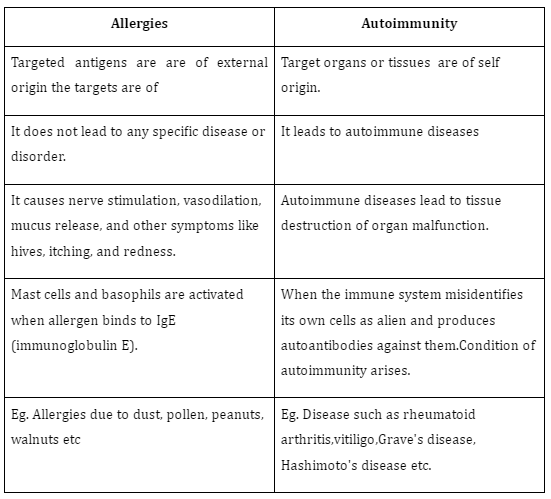
Difference between Allergies and Autoimmunity
Summary
Humans are endowed with a powerful immune system that can recognise and eliminate potentially dangerous chemicals from the body. The battle goes beyond mere eradication; it also records the antigen in the body’s memory, making it capable of withstanding future attacks. The immune system’s effectiveness can be compromised by autoimmune and allergic illnesses. Immune reactions are triggered in allergic people by ordinary items that are typically harmless. When the body targets its own tissues and organs thinking they are foreign objects it leads to autoimmune diseases .
Frequently Asked Questions
1. What is Anaphylaxis?
Ans: An unexpected emergency known as anaphylaxis can cause due to an allergen and lead to severe throat swelling, eye irritation, breathing and swallowing difficulties etc.
Due to a quick reduction in blood pressure, some persons under an anaphylactic attack feel dizzy.
2. How are allergies identified?
Ans: A skin test and blood test are used to diagnose allergies. An allergen drop is applied to the skin and pricked softly to perform a skin prick test. A blood test counts all IgE antibodies produced in response to a specific antigen.
3. What effect do immunosuppressants have on autoimmune disease patients?
Ans: An immune reaction against self-tissues is elicited by an overreacting immune system in an autoimmune disease patient. Immunosuppressants lower the body’s immunological response, but they can have negative side effects include a higher risk of infection.