Introduction
The air is a fundamental element of planet Earth that sustains life. The broad term “air” is used to describe the mixture of gases that makes up the earth’s atmosphere. It is a clear gas required for breathing and performing regular cellular activities. Air is a very essential and makes up the atmosphere of the earth.
It has the following other applications-
- All life depends on air to thrive, including humans, plants, animals, and other species.
- The air is necessary for the water cycle to take place.
- It facilitates combustion and breathing.
- It keeps the temperature constant.
- Air assists in the process of pollination in wind-pollinated plants.
Components of Air
The air around is composed of various components given below-
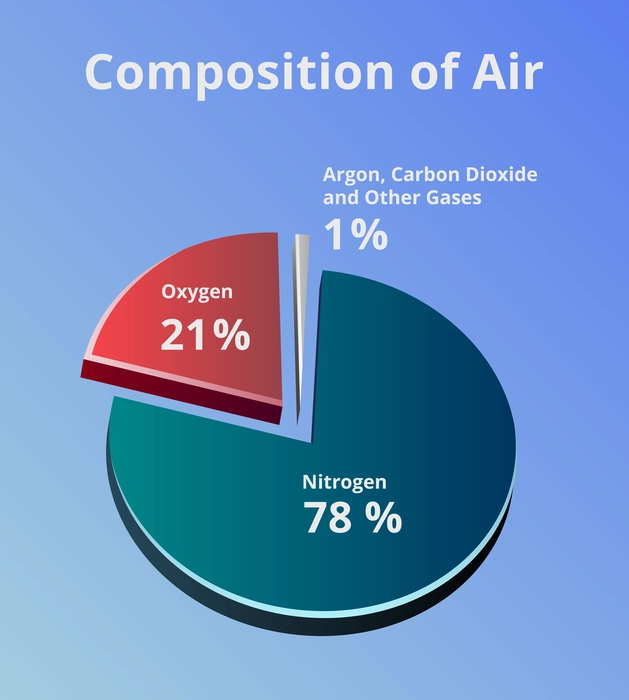
- Oxygen makes up around 21% of the air.
- The highest amount of gas present in the air is Nitrogen, which makes up 78% of the total air.
- Argon is 0.9% of the total air.
- Carbon dioxide is the lowest around 0.04% of the air.
- There are still other gases which are present in very lower concentrations eg. water vapour.
- Microscopic airborne particles known as “aerosols” are also present in the air and are present in minute quantities.
- These aerosols include bacteria, suspended dust, pollen, and spores.
Properties of Air
- Air is colourless and odourless and cannot be seen, heard or touched.
- It is a mixture of many gases and they occupy space and matter.
- Air exerts pressure. Near the surface, the air pressure is more and at higher altitudes the air pressure is low.
- When heated the air expands and when cooled the air compresses.
Uses of Air
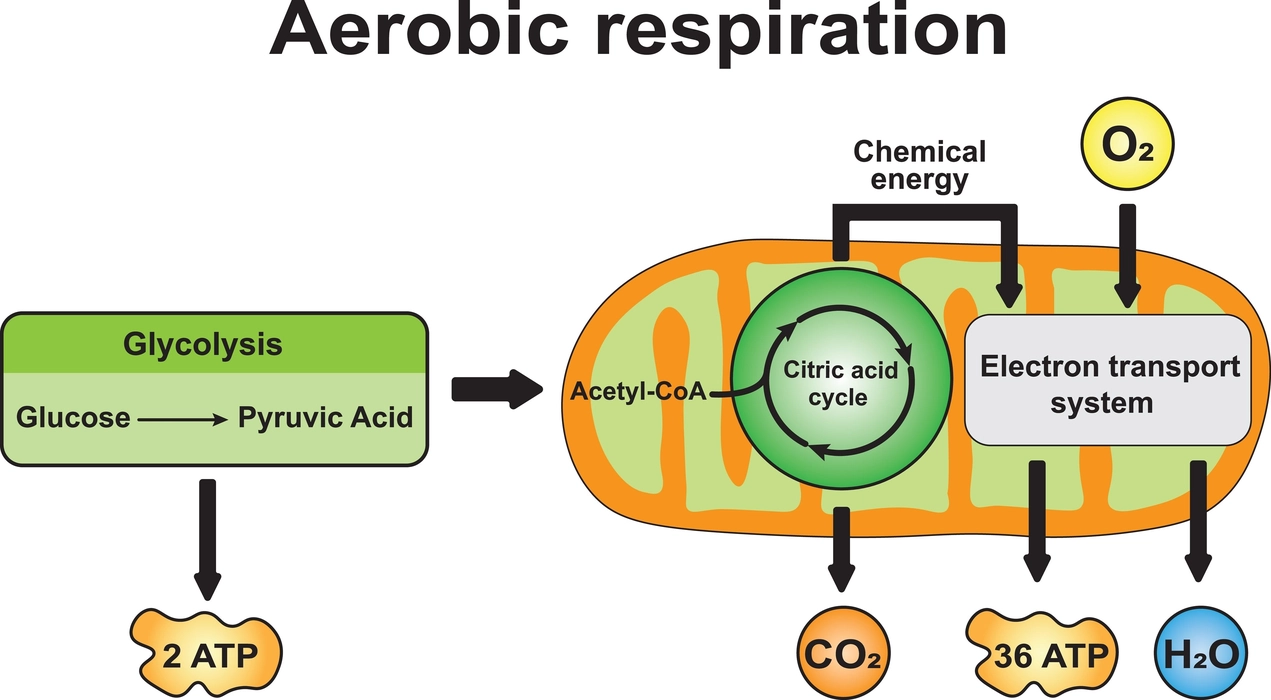
Respiration
- Respiration is the process where gaseous exchange occurs and oxygen is inhaled and carbon dioxide is exhaled.
- The two main gases involved in respiration are carbon dioxide and oxygen.
- Plants and animals require oxygen to convert the chemical energy found in food into energy that can be used for various metabolic processes.
- This energy is used in all actions of growth, development, locomotion and reproduction.
- Oxygen is created through the process of photosynthesis, which occurs when plants use carbon dioxide to make chemical energy while utilizing light energy.
Combustion
- A fuel oxidises when it is burned and produces lots of energy.
- This is an exothermic reaction wherein light and heat are generated.
- Any carbon-containing substance that is burned in the presence of oxygen produces carbon dioxide, water vapour, heat, and light energy.
- Colourful flames are created when methane, an essential element of combustion, combines with air. These colourful flames are an indicator of the combustion reaction.
- Explosive burning might occur if there is too much oxygen present hence to prevent this nitrogen gas is present in the atmosphere.
- Nitrogen does not contribute to combustion and inhibits too much oxygen from causing higher reactivity. Therefore, these two components work together to make sure that fuel energy is used in a controlled way.
- The heat that is generated during the process of combustion is used to cook, run our vehicles, generate electricity etc.
Regulation of temperature
- The earth’s surface is kept at a constant temperature by air.
- The density of hot air is less and hence it rises above the ground. This leads to the formation of a low-pressure area which is quickly filled by cool air.
- This phenomenon leads to the formation of winds.
- As the temperature of the air rises the air moves up draws in cooler air from the surroundings, warms it up, and the cycle repeats.
- When hot air rises, it radiates heat into space before sinking back to earth.
- Convection is the process of moving heat, and this is referred to as temperature regulation.
- Heat is transferred in this way from hotter to colder places and thus the temperature of the earth is regulated.
- The atmosphere and air also help to cool the earth and protect it from the sun’s excessive UV rays.
Summary
Air surrounding the earth makes up its atmosphere. The air is a mixture of gases and is essential to many living things. Air consists of 78% of nitrogen, 21% oxygen, 0.9% argon, and 0.04% carbon dioxide, and there are traces of other gases as well. The thick layer of air supports vital life-supporting activities. Contrarily, air is a substance and it has mass, can be compressed, and takes up space. Air performs the following major processes—breathing, combustion, and regulating the earth’s temperature.
Frequently Asked Question
1. Give the function of the ozone layer
Ans: The ozone layer, which is found in the stratosphere of the earth and absorbs the majority of the sun’s ultraviolet rays, works as a screen to protect the planet from these rays.
2. What does acid rain mean?
Ans: Polluted air consists of oxides of nitrates and sulphates. These oxides react with water vapour and other air components to form sulfuric acid and nitric acid. When there is rainfall both of these acids fall on earth which is termed acid rain. This acid rain is not only harmful to people but also affects various other living organisms.
3. What are the ill effects of air pollution?
Ans: Air pollution is a very severe problem that aggravates pre-existing respiratory and cardiac problems and causes several pollution-related ailments. Common diseases caused due by air pollution are lung cancer, stroke, chronic obstructive pulmonary disease (COPD), and respiratory infections.