Introduction
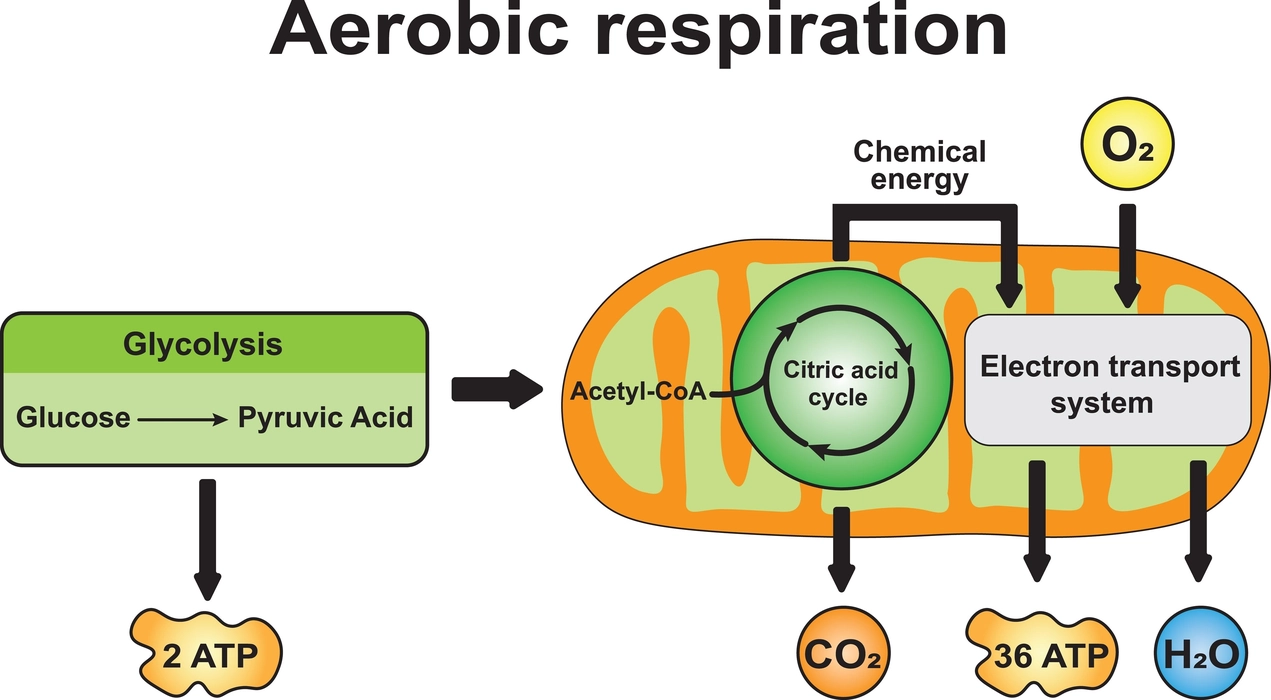
All of the body’s cells require energy to support various metabolic processes, thus every living thing engages in cellular respiration to release energy, which is then stored in the form of ATP. After ingestion, food is transported into the stomach via the oesophagus, where stomach acids and enzymes break it down into several smaller bits, including glucose. Since glucose is the most prevalent monosaccharide and the first substrate for the metabolism of carbohydrates, where it is broken down to release energy, glucose and ATP are the molecules that carry the energy.
Definition of Cellular Respiration
All plant and animal cells produce energy through a process called cellular respiration (excluding RBCs). Food glucose is broken down into carbon dioxide, water, and energy with or without oxygen throughout this process. As a result, it liberates ATP and releases carbon dioxide as a waste product (adenosine triphosphate).
Difference between Respiration and Breathing
| Respiration | Breathing |
| Respiration is the physiological process of breathing in and breathing out. Energy is released from cells during the chemical breakdown of food-derived glucose. | Breathing is the movement of oxygen into the body from the outside environment and the release of carbon dioxide from the lungs into the outside environment. |
| It is categorised into cellular respiration and physiological respiration. | Since breathing is a form of respiration, it is often referred to as physiological respiration. |
| In cells, and notably in cellular organelles like the cytosol and mitochondria, cellular respiration takes place. | It takes place in the lungs. |
| There is the involvement of enzymes. | There is no involvement of enzymes. |
| It produces ATP that is converted into energy. | It does not produce energy. |
Glycolysis
- One glucose molecule is broken down into two pyruvate molecules in this process, which also results in the creation of ATP.
- Every cell in the body contains it in the cytoplasm. Hexokinase enzyme converts glucose to glucose-6-phosphate.
- By using phospho-hexose isomerase, which are isomers of one another, glucose 6-phosphate is converted to fructose 6-phosphate.
- By phosphorylating fructose 6-phosphate, phosphofructokinase catalyzes the irreversible conversion of fructose 6-phosphate to fructose 1,6-bisphosphate.
- Aldolase catalyzes the breakdown of fructose 1,6 bisphosphate into glyceraldehyde 3-phosphate and dihydroxyacetone phosphate.
- The reversible interconversion of glyceraldehyde 3-phosphate and dihydroxyacetone phosphate is carried out by phosphotriose isomerase.
- Glyceraldehyde 3-phosphate is converted to 1,3-bisphosphoglycerate by glyceraldehyde 3-phosphate dehydrogenase.
- In this stage, \(NA{D^ + }\) is converted to \(NAD{H^ + }\) and \({H^ + }\), which adds a phosphate group to glyceraldehyde 3-phosphate. With the creation of ATP, the enzyme phosphoglycerate kinase converts 1,3-bisphosphoglycerate into 3-phosphoglycerate.
- Phosphoglycerate mutase converts 3-phosphoglycerate into 2-phosphoglycerate, and these two substances are isomers.
- Enolase transforms 2-phosphoglycerate into the highly energetic molecule phosphoenolpyruvate once water is removed.
- In the presence of pyruvate kinase, phosphoenol pyruvate is transformed into pyruvate along with the creation of ATP.
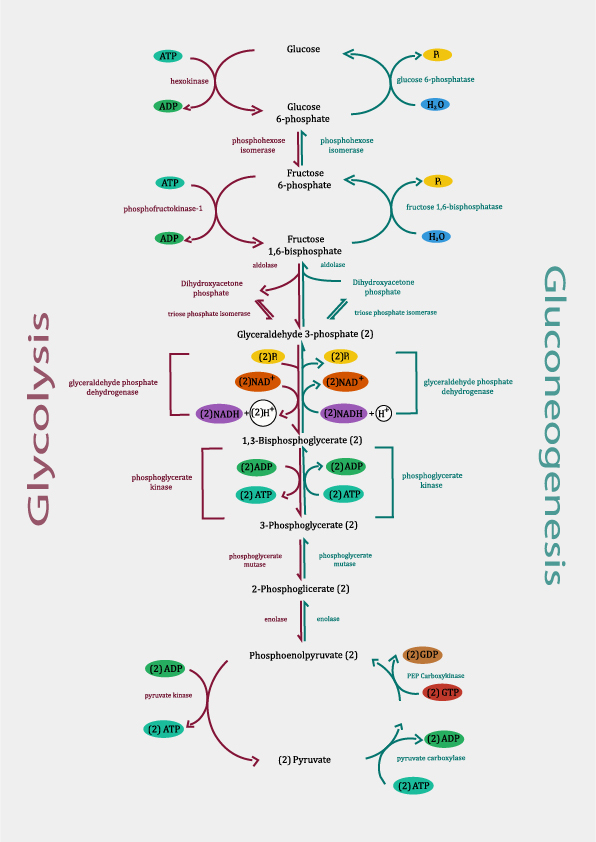
Generation of ATP: Two pyruvates, two NADH, and two ATP molecules are the final products of glycolysis. Due to the conversion of glucose into two pyruvates, 8 ATP molecules are produced.
Krebs Cycle
Acetyl CoA is converted into carbon dioxide and water by some chemical processes called Krebs cycle.
- Pyruvate is converted to acetyl CoA through oxidative decarboxylation by pyruvate dehydrogenase.
- The elimination of carboxylate groups to create carbon dioxide is known as oxidative decarboxylation. Acetyl CoA and oxaloacetate are condensed by citrate synthase.
- Aconitase converts citrate into isocitrate.
- Isocitrate dehydrogenase uses oxidative decarboxylation to change the isocitrate to oxalosuccinate, which is then transformed into -ketoglutarate.
- By removing the carboxylate group from ketoglutarate and generating carbon dioxide, the enzyme ketoglutarate dehydrogenase transforms ketoglutarate to succinyl CoA.
- Succinate thiokinase causes succinyl CoA to be converted to succinate. A phosphorylated group is added to GDP to create GTP, which is then converted into ATP by a protein called nucleoside diphosphate kinase.
- By catalysing the conversion of succinate to fumarate and producing \(FAD{H_2}\), succinate dehydrogenase.
- By including water, fumarase catalyses the conversion of fumarate to malate.
- Malate dehydrogenase converts malate to oxaloacetate and generates NADH in the process.
- The cycle is maintained by mixing the oxaloacetate with more acetyl CoA molecules.
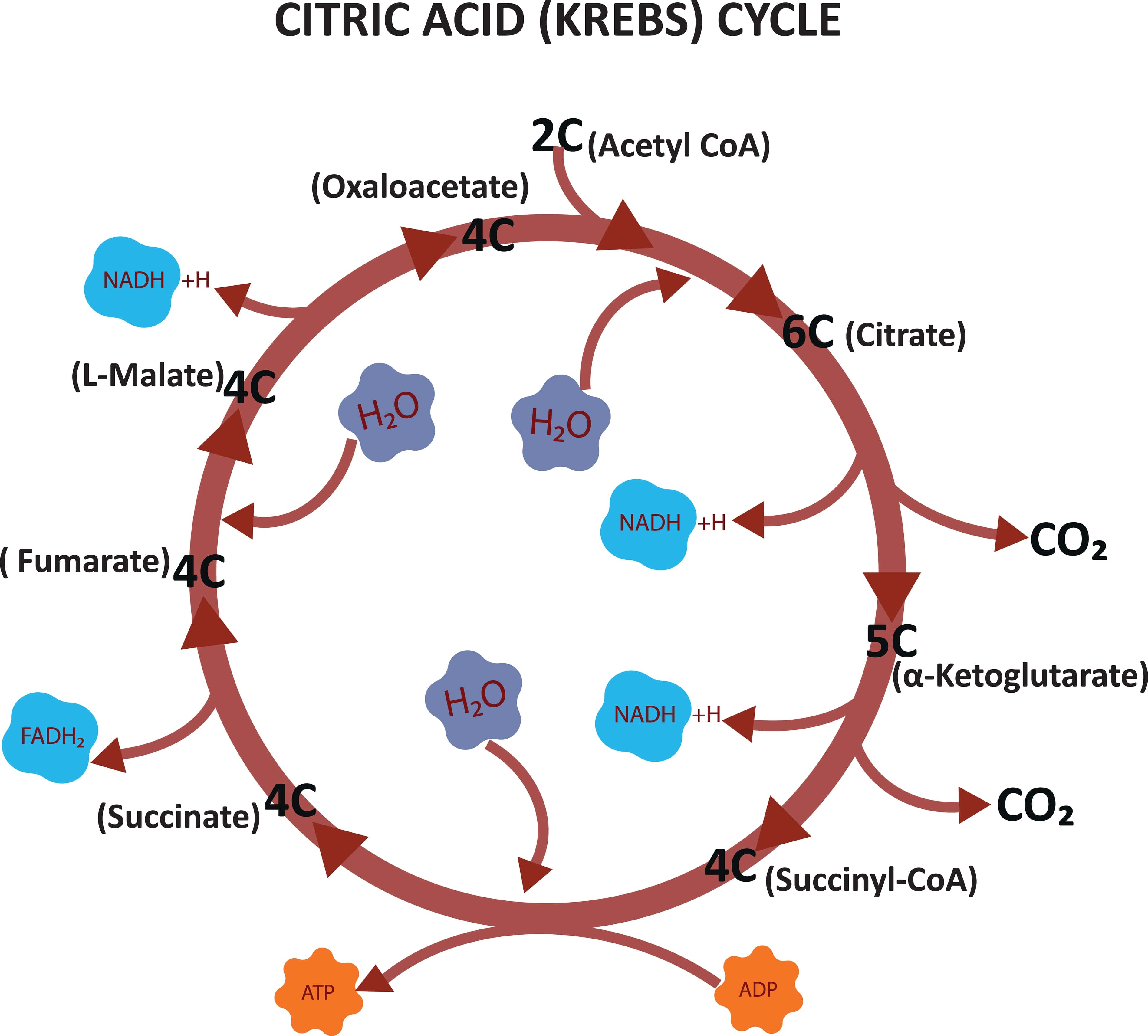
Generation of ATP: In the Krebs cycle, 12 ATP is produced as a result of the production of 2 \(C{O_2}\), 3 NADH, and 1\(FAD{H_2}\).
Electron Transport Chain or Terminal Oxidation or Oxidative Phosphorylation
The proton gradient created by the electron transport chain (ETC), a chain of proteins that transports electrons through the mitochondrial membrane, powers ATP generation. A series of ETC enzyme complexes:
- NADH-ubiquinone reductase – Complex I
- Succinate CoQ reductase – Complex II
- Ubiquinone-cytochrome c oxidoreductase – Complex III
- Cytochrome oxidase – Complex IV
- ATP synthase – Complex V
Through electron carriers such as flavoproteins, cytochromes, coenzyme Q, nicotinamide nucleotides, and iron-sulfur proteins, these catalyze the transport of electrons.
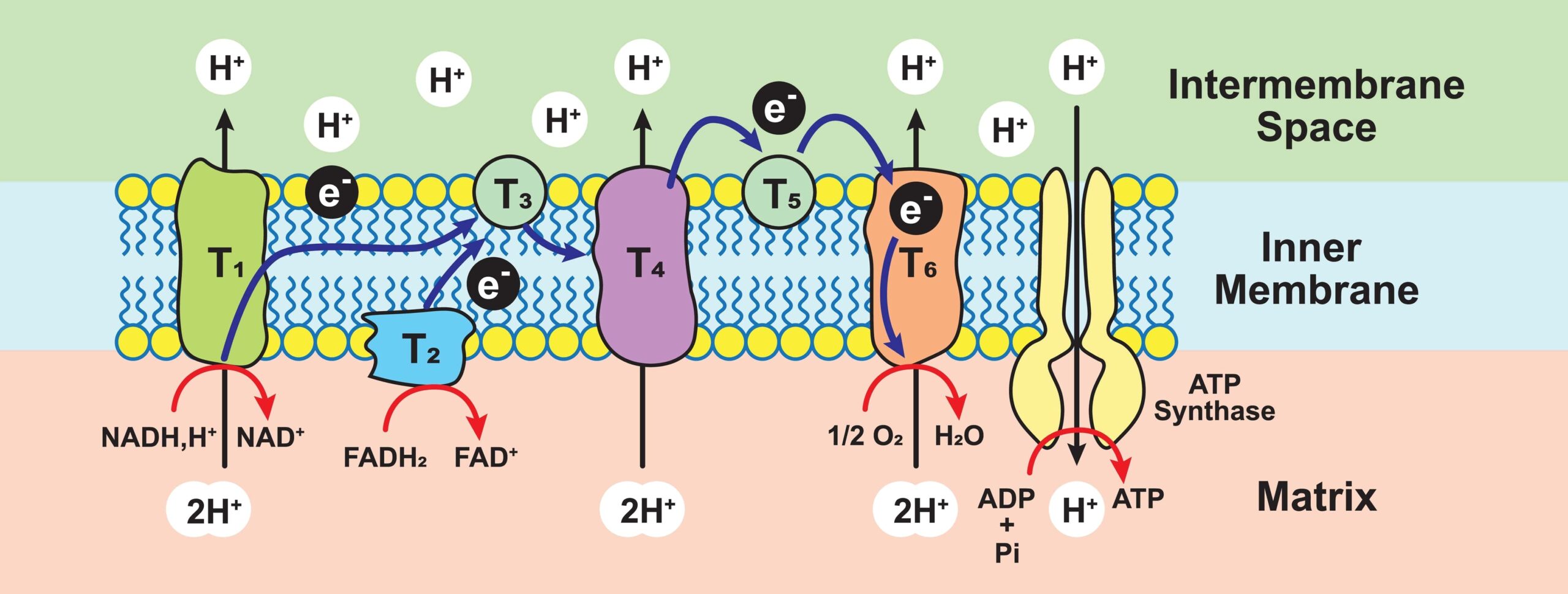
Since energy is lost during the passage of electrons through ETC, the ATP synthase complex uses the energy to produce ATP from ADP, a procedure known as oxidative phosphorylation. 32 ATP molecules are generated during oxidative phosphorylation and ETC.
Differences between Glycolysis and Krebs Cycle
| Glycolysis | Krebs cycle |
| It involves both aerobic and anaerobic respiration. | It involves only aerobic respiration. |
| The substrate substance is glucose. | The substrate material is acetylAcetyl CoA. |
| Glycolysis takes place in the cytoplasm. | The KrebsKrebs cycle takes place in the mitochondria. |
| It consumes two molecules of ATP. | It does not consume ATP. |
| Carbon dioxide is released in glycolysis. | Carbon dioxide is not released in the Krebs cycle. |
| It is a linear enzymatic reaction. | It is a non-linear pathway. |
| It occurs in both eukaryotes and prokaryotes. | It occurs in eukaryotes. |
Difference between Aerobic and Anaerobic Respiration
Summary
All plant and animal cells produce energy through a process called cellular respiration. Due to the conversion of glucose into two pyruvates, 8 ATP molecules are produced in glycolysis. In the Krebs cycle, 12 ATP is produced as a result of the production of 2 \(C{O_2}\), 3 NADH, and 1 \(FAD{H_2}\). 32 ATP molecules are generated during oxidative phosphorylation and ETC.
Frequently Asked Questions
1.How do electron carriers function?,
Ans. The metabolite is present at one end and oxygen is at the other end, therefore the electrons are carried by a series of proteins.
2. What are the processes in the conversion of glucose to pyruvate that require energy?
Ans. By using enzymes in intermediary processes, glyceraldehyde 3-phosphate and dihydroxyacetone phosphate are produced from glucose. These processes call for energy.
3. What is oxidative phosphorylation?
Ans. Oxidative phosphorylation, which takes place in the mitochondria, is the addition of the phosphate group through reactions that use the energy produced when ATP is made from ADP.
4. What is the importance of cellular respiration in living organisms?
Ans. Energy is released during cellular respiration, which activates a number of bodily processes. Therefore, ensuring the survival of living things is important.