Introduction
Animals have sophisticated nervous systems. It is a vital system that controls all of our body’s voluntary and involuntary movements. The nervous system controls all complicated functions, including reading, remembering, feeling emotions, and logical thought. The brain, spinal cord, and nerve network are all parts of the nervous system.
Together, these organs carry out the complex functions of the nervous system.
Nervous System Classification
The nervous system is classified into 3 types i.e the Central nervous system(CNS), the Autonomic nervous system(ANS), and the Peripheral Nervous System (PNS).
Central nervous system(CNS)
- The spinal cord and brain together make up the CNS.
- The brain is covered by a fluid known as a Cerebrospinal fluid(CSF).
- Three membranes—the dura mater, arachnoid, and pia mater—cover the brain.
- Humans are made up of the forebrain, midbrain, and hindbrain.
- The forebrain is made up of the diencephalon and cerebrum and is further divided into the dorsal thalamus and ventral hypothalamus.
- The cerebrum is in charge of our motivation, reasoning, imagination, memory, thinking, and consciousness.
- The midbrain is made up of four circular structures called the cerebral aqueduct and corpora quadrigemina.
- The hindbrain is made up of the cerebellum, medulla oblongata, and pons.
- All voluntary motions and body equilibrium are controlled by the cerebellum, whereas the pons regulates breathing and the sleep cycle.
- The medulla oblongata regulates breathing, circulation, gastric secretions, vomiting, and salivation.
- The spinal cord is a cylindrical structure that resides in the neural canal of the vertebral column.
- The spinal cord is the location where all the nerves join and the information is sent to the brain.

Autonomic nervous system(ANS)
- ANS is known as the Autonomic Nervous system.
- It regulates the body’s internal organs’ involuntary functions.
- The sympathetic and parasympathetic nervous systems are the two types of ANS.
Peripheral Nervous System (PNS)
- The PNS is made up of nerves that emerge from the spinal cord and brain.
- The term “cranial nerves” refers to nerves that are emerging from the brain.
- Spinal nerves are nerves that arise from the spinal cord.

Parts of the nervous system
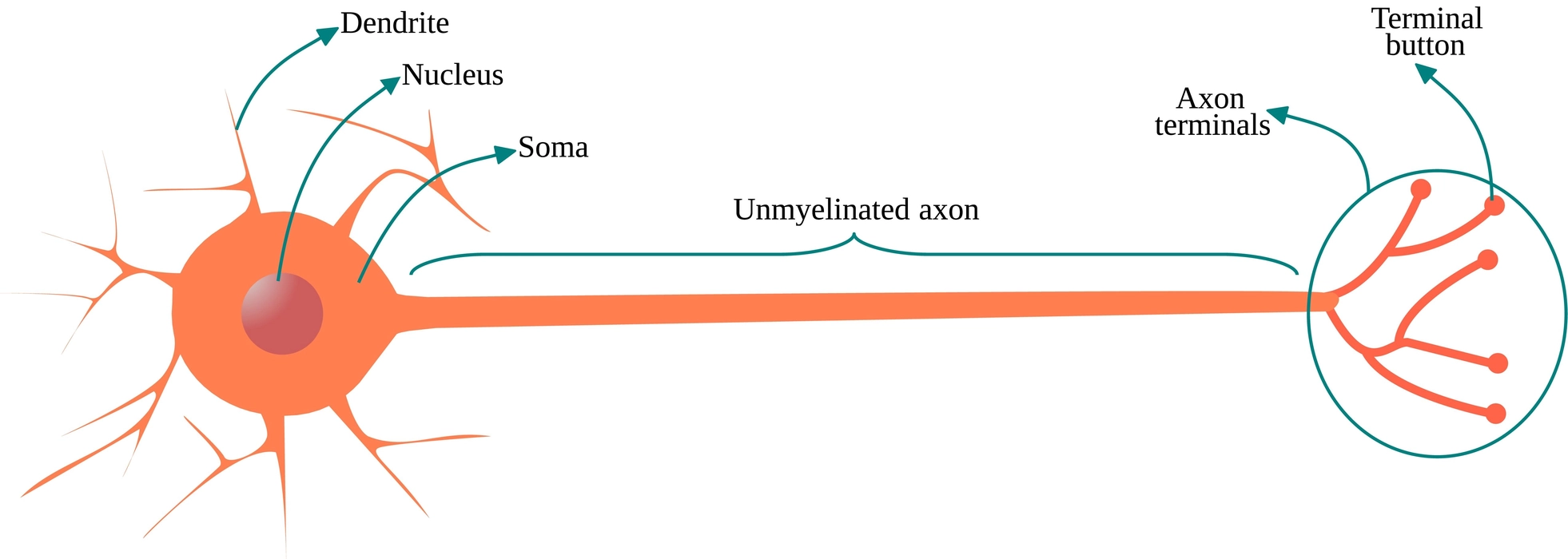
Neurons are the structural and functional components of the nervous system. Neurons are typically referred to as nerve cells. It is the longest cell in the human body and sends electrical impulses as messages.
Following are the parts of a neuron.
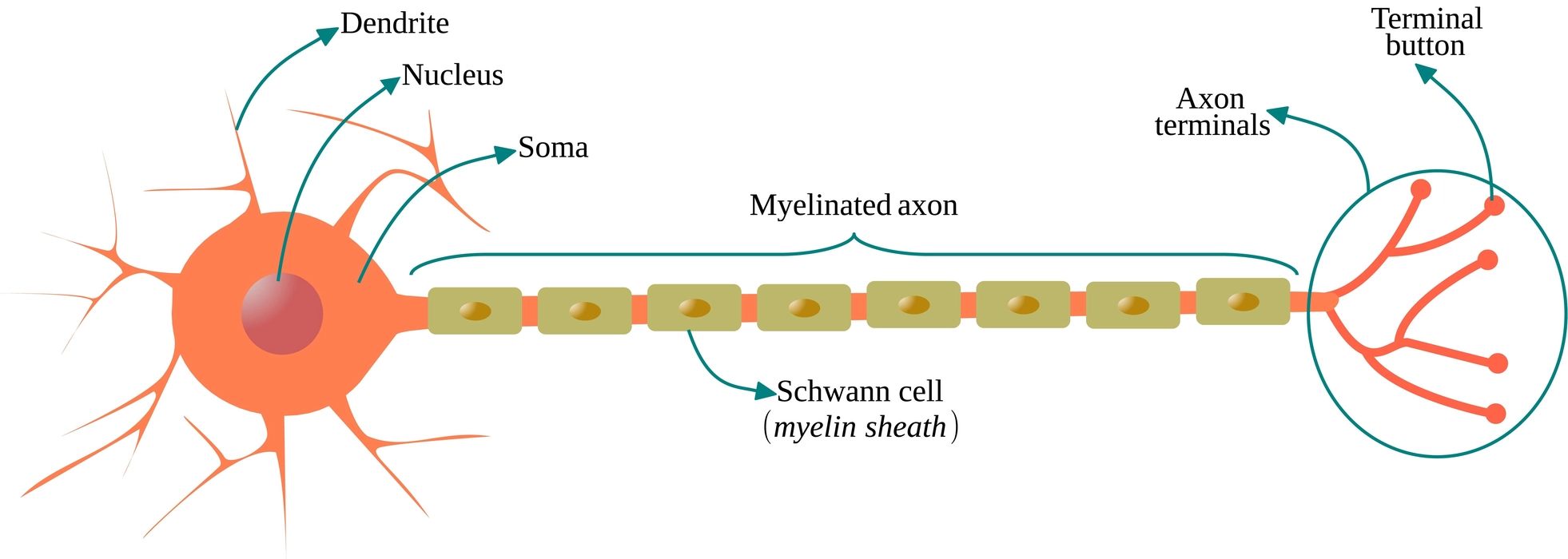
- Cyton: The cell body is the main component of the neuron. The cyton contains cell organelles, a nucleus, and cytoplasm.
- Dendrites: These cytoplasmic extensions that protrude from the cell body are numerous and highly branched. They carry electric impulses both inside and outside of the cell body.
- Axon: It emerges from the cyton as a solitary, unbranched cylindrical projection. A myelin sheath protects the axon. It transports impulses away from the cyton.
Functions of the nervous system
- The network of neurons is in charge of message reception and transmission, which in turn controls and coordinates the various bodily parts.
- The cerebrum is in charge of our intelligence, thinking, consciousness, memory, imagination, reasoning, and willpower.
- The nervous system also controls voluntary movement and body balance. The cerebellum is in charge of this activity.
- The spinal cord is involved in the reflex action and helps in quick response during dangerous situations.
- The Nervous system of the body is responsible for controlling all automatic reactions to peripheral nerve stimulation such as breathing, stomach secretion, vomiting, etc.
Types of nerves
Classification of nerves is based on function, structure, and myelin sheath.
Classification based on function
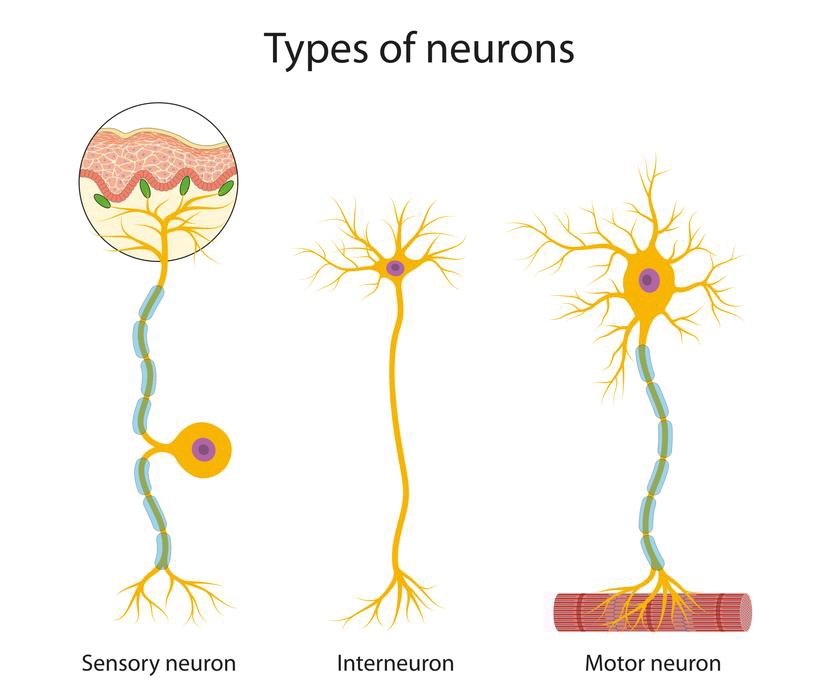
- Afferent or Sensory Neurons: These nerves perform the function of carrying impulses to the Central Nervous system from the various sense organs such as eyes, nose, skin, etc.
- Efferent or Motor Neurons: These nerves perform the function of carrying impulses from the Central Nervous system to various effector organs such as muscles, organs, etc.
- Interneurons or Association Neurons: This neuron help in transferring impulses between the Sensory and Motor neurons.
Classification based on the structure
- Unipolar Neurons: In these neurons, the cell body gives rise to only a single nerve process that performs the function of both axon and dendron. These are seen in the spinal and cranial nerve ganglia.
- Bipolar Neurons: Here, two nerve processes are formed from the cell body. One of these becomes the axon and the other becomes the dendron. These are found in various sensory organs such as the nose, tongue, eye, etc.
- Multipolar Neurons: Multiple dendrons are generated from a single-cell body. These are the most common types of neurons found in the nervous system.

Classification based on the myelin sheath
- Myelinated nerve fiber: A myelin sheath protects the axon
- Non-myelinated nerve fiber: The nerve fiber here is not protected by the myelin sheath.
Summary
The human nervous system is the body’s most complicated network system. It is made up of the autonomic, peripheral, and central nervous systems. It enables us to carry out routine tasks, difficult activities, and bodily functions. With the aid of a sophisticated network of nerves, the nervous system functions as a communication network that sends and receives signals from the brain and spinal cord to every area of the body. The nervous system is made up of neurons and based on their structures, functions, and whether or not myelin sheaths are present, neurons are further classified.
Frequently asked question
1. What is the Cerebrospinal fluid?
Ans: The brain’s ventricles (hollow spaces) are lined with tissue. This tissue produces cerebrospinal fluid. This fluid circulates around and inside the brain and spinal cord to nourish and protect them.
2. What signs of a weak neurological system?
Ans: Symptoms of disorders of the nervous system are-
- headaches that develop suddenly or persistently.
- tingling feeling or loss of sensation.
- loss of muscle strength or weakness.
- eyesight loss or double vision
- memory deterioration
- reduced mental capacity
3. Which components have a negative impact on the nervous system?
Ans: Following have a negative impact on the nervous system
- Trauma (injuries), particularly spinal cord and head trauma.
- Issues that exist from birth (congenital).
- Mental health issues such as depression, psychosis, or anxiety disorders.
- Exposure to poisons like lead, arsenic, or carbon monoxide.