Introduction
Environmental changes are natural and will continue to occur from time to time. Change can be abrupt or gradual, like climate change and global warming. Because of the changing environment, some creatures become extinct while certain species can thrive with very minor alterations. These modifications are passed down across generations, enabling the species to adapt to its environment and increase in population. These modifications are known as adaptations. A natural environment where a species grows and reproduces is known as a habitat.
Depending upon various habitats there are the following adaptations–
For more help, you can Refer to Lesson 9 – Living things and habitat in Science Class 6. Check out the video Lesson for a better understanding.
Adaptations in aquatic habitats
Numerous elements, such as light penetration, water composition (nutrient and salt content), oxygen availability, pressure, etc, are various factors that affect life in the aquatic environment. For these purposes, plants and animals are adapted in the following ways-
Plant adaptations-
- Aquatic plants have tissues called aerenchyma to move oxygen from exposed surfaces to low-oxygen submerged sections.
- Aerenchyma also provides buoyancy to the plant and helps it float and helps to absorb natural light and oxygen.
- The root system is essentially nonexistent because water is all around them. If existent, it is rather small and mostly used for anchorage.
- To endure water currents leaves on submerged plants are typically tiny or ribbon-shaped.
- The stems are tall, hollow, light, and slender.
- Examples include water lilies, sea grass, and lotus.

Animal adaptations-
- Aquatic animals use their gills to breathe.
- Syphons are breathing tubes used by aquatic insects to draw oxygen from the air when submerged in the water. While some creatures, including salamanders, breathe through the skin.
- The blow holes on larger animals like whales and dolphins allow them to take in oxygen as they ascend to the surface and expel carbon dioxide.
- Fish have streamlined bodies to lessen swimming-related water resistance.
- Fins aid in swimming and tails act like rudders to maintain direction.
- Dolphins, whales, fish, etc. are a few examples.

Adaptations in desert habitat
Deserts are dry areas that only get a little rain occasionally throughout the year. It has an arid climate and rocky, sandy soils. Deserts face high temperatures and intense sunlight. For these purposes, plants and animals are adapted in the following ways-
Plant adaptations-
- A common desert adaptation is to conserve body fluids and prevent water loss through transpiration. To stop water loss through transpiration, desert plants’ leaves have thick, waxy cuticles.
- Due to the poor external availability, the stems are fleshy and store a lot of water.
- Desert plants have spines as a kind of defense against herbivores.
- To access deep subterranean water resources, plants have long, deep roots.
- Cacti, and agave, are some examples of desert plants.

Animal adaptations-
- The temperature in the desert is usually very high, hence small organisms have evolved nocturnal behaviors to ensure their survival. At night, when temperatures are generally low, they hunt for prey.
- Many desert animals have skin that is made to keep water from evaporating, and their bodies store lipids which can help them endure prolonged hunger periods.
- High-speed winds frequently carry small sand particles; these animals have large eyelashes, hairy ears, and tight nostrils which prevent sand from getting into their delicate bodily parts.
- Desert animals include kangaroo rats, camels, and desert cats.

Summary
Every living thing is constantly interacting with its environment. The percentage of survivors they have will determine how long the species will exist. Changes in the environment might be gradual or abrupt. However, organisms—whether they be plants or animals—develop specific inherited traits that enable them to adapt to environmental changes. We refer to them as adaptations. Desert-dwelling organisms have developed adaptations that reduce body water loss. Water-dwelling organisms have developed adaptations that help them breathe and adjust to the water pressure. Adaptations help organisms function better and are essential for survival.
Frequently Asked Questions
1. Give some common examples of adaptations.
Ans: Some examples of adaptations are-
- Pointed teeth and claws of carnivores.
- Varied beak shapes to obtain food.
- Webbed feet of ducks.
- Leaves thick cuticles in xerophytic plants.
- White color fur in arctic animals prevents predation.
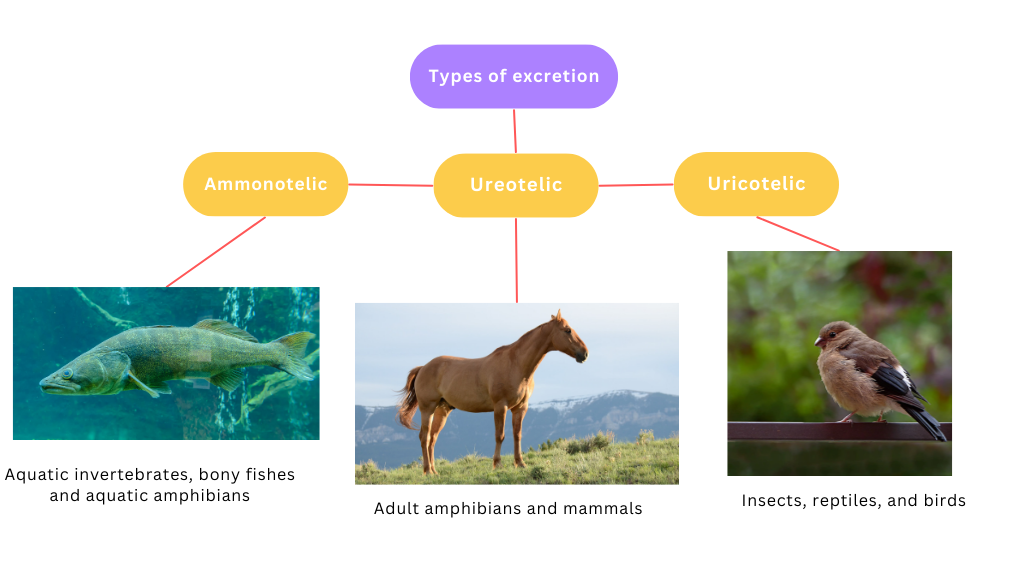
- Uricotelism in xerophytic animals.
2. What are Physical, physiological, and Behavioural adaptations?
Ans: Physical adaptations are characteristics that organisms have acquired as a result of their environment. To live in their ecological niche, organisms engage in internal functional mechanisms known as physiological adaptations. Behavioral adaptations are the behaviors or reactions the organism does in response to its surroundings.
3. Describe camouflage.
Ans: The physical adaptation of creatures to resemble or blend into their surroundings to survive attacks from predators is called camouflage.








 Excretion
Excretion