Introduction
Various optical systems, including lenses, mirrors, and spherical mirrors, reflect or diverge light to create various kinds of images. Rules of reflection and laws of refraction are the two fundamental laws that govern picture generation. In the creation of images, these laws are crucial. Different-sized pictures are created at various positions when light is reflected by lenses or mirrors. It can vary in size from very little to extremely large to the same size as the thing, and it can also become blurry at times. Consequently, the creation of the hazy image is referred to as an abbreviation. A hazy image is created when light rays diverge after passing through a lens in a particular spot. This is known as an aberration of the lens.
Lenses and curved mirrors are two objects that can induce optical aberration. This phenomenon causes a deviation in the light rays. Not all of the light’s beams concentrate on the focal point. By virtue of optical aberration, images are essentially blurry.
Types of Aberration
For a single wavelength light, there are five types of aberration.
- Spherical aberration
- Coma
- Astigmatism
- Curvature of field
- Distortion
Let our expert guru Mr. Mayur be your guide toward improving your understanding of this chapter better. Watch the related video of this chapter in Class 7th Science lesson no-15
1. Spherical aberration
It is a defect of the lens due to which the light beam does not concentrate at a single point, as a result, the exterior part of the image gets blurred. This aberration is caused in lenses or mirrors when light beams fall on the exterior part and concentrate at different points.
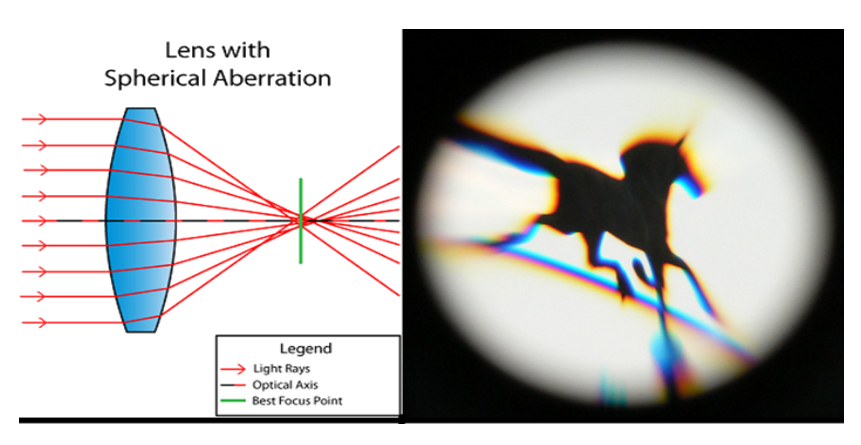
Correction of the spherical aberration
- Spherical aberration can be corrected by using a mirror of different non-spherical shapes. A parabolic mirror or an ellipsoidal mirror can replace a spherical mirror.
- Spherical aberration can be removed by blocking the marginal rays. A circular annular mask on a lens can remove the marginal rays.
- By using suitable radii of curvature of the two surfaces of a lens the spherical aberration can be minimised.
- A combination of concave and convex lenses can also reduce the spherical aberration.
2. Coma
The coma is a type of spherical aberration. It occurs due to the variation in the magnification of the image. The rays that are coming from an off-axial object and the rays pass through different circular zones of the lens. Due to this the image will appear in a comet-like shape. So, this aberration of the lens is known as a coma.
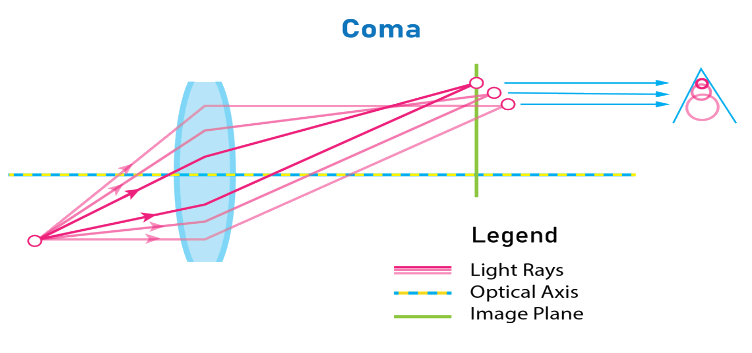
Types of coma
The coma varies due to the magnification of different zones of the lens. There are two types of coma
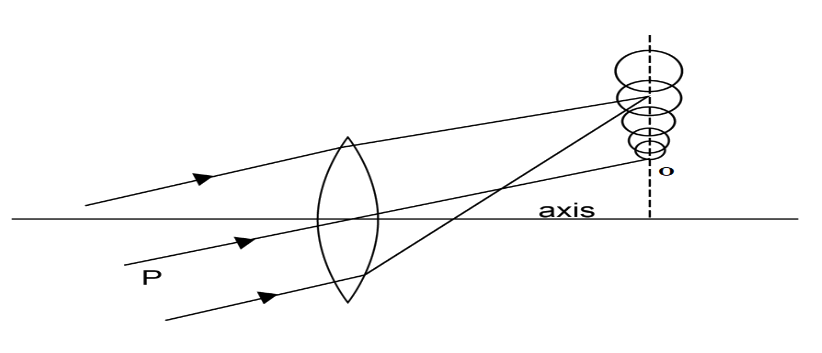
1. Positive coma
When the lateral magnification of the outer zone is greater than the central zone, then the coma is said to be a positive coma.
2. Negative coma
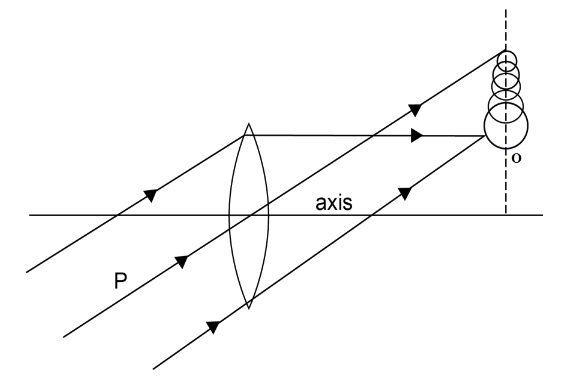
When the lateral magnification of the outer zone is smaller than the central zone, then the coma is said to be a positive coma.
Correction of coma aberration:
- By bending the lens the coma can be corrected but not completely processed will only work in the case of a single lens.
- The coma can be corrected by the combination of lenses that are symmetrical and in the application of curvature type of lenses surface
3. Astigmatism
It is the most common defect that occurs in the eyes. In this defect, the front part of the lens or cornea has an irregular curve. So, when the light refracts by the retina it forms a blurry image. Astigmatism is caused when eyelids put pressure on the cornea. mostly these defects passed over generations from their parents.
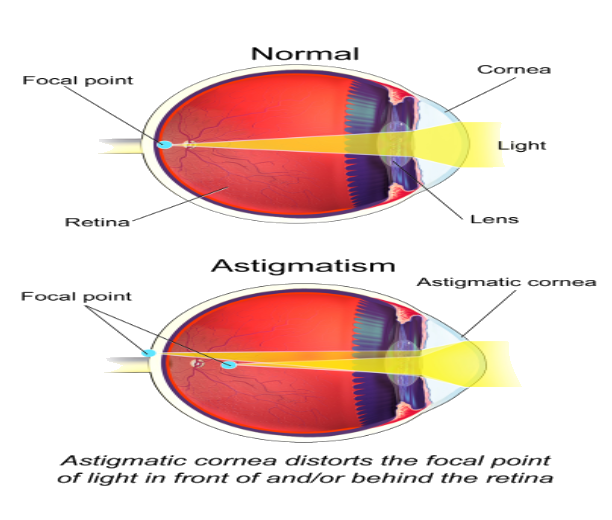
Types of Astigmatism
There are two types of astigmatism.
1. Positive astigmatism: This is an abbreviation for which a person suffered from a farsightedness defect of the eye. For convergent lenses, the transverse focal is greater than the meridional focal length. This type of astigmatism is known as positive astigmatism.
2. Negative astigmatism: This is an abbreviation for which a person suffered from a near sightedness defect of the eye. For divergent lenses, the sagittal focal is smaller than the meridional focal length. This type of astigmatism is known as negative astigmatism.
Correction for astigmatism aberration:
- The combination of concave and convex lenses can also reduce astigmatism.
- Using lenses with different radii of curvature helps to reduce astigmatism.
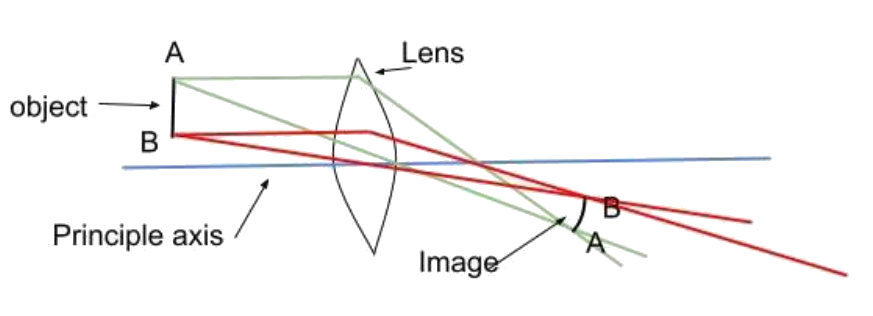
4. Curvature of Field
When the central part of the image is flat but the exterior part of the image is curved concerning the object then this type of aberration in the image is called curvature of field. The curvature of the field arises when the paraxial focal length is greater than the marginal focal length.
Correction for the curvature of field:
- The condition for no curvature is known as the Petal condition. When the image of the plane object projects on the Petal surface the aberration gets reduced.
5. Distortion
The type of aberration in which the shape of the image gets distorted in the transverse plane as a whole is known as distortion. It arises due to the variation in the lateral magnification with the lateral distance of an object from the lens distance.
Types of distortion:
There are two types of distortion
1. Barrel distortion: If magnification decreases with axial distance then the image of a square takes the form resembling a barrel. This type of distortion is known as barrel distortion.
2. Pincushion distortion: If the magnification increases with lateral distance, then a square’s image takes a shape like a pin-cushion. This type of distortion is known as pin-cushion distortion.
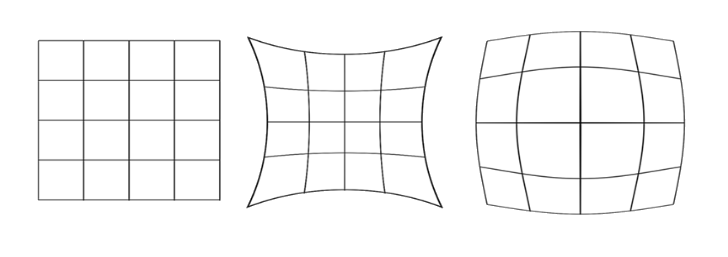
Correction of the distortion:
- A combination of two lenses can help us to avoid distortion.
- Using both barrel distortion and pincushion distortion combination to cancel out each other’s effect.
6. Chromatic aberration
When the images are formed by the refraction of white light, As a result, the image becomes colored. This type of defect in the image is known as chromatic aberration. This phenomenon occurs due to the dispersion of the white light.
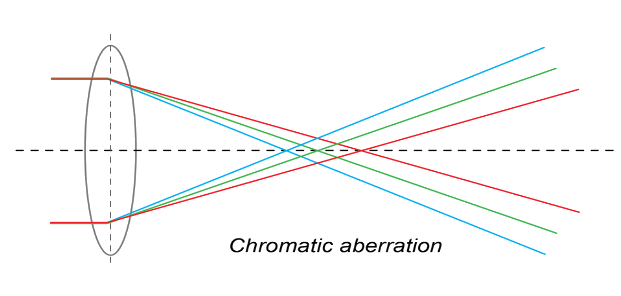
Types of chromatic aberration:
There are two types of chromatic aberration
1. Lateral chromatic aberration: In this aberration, all the light rays concentrate at the same plane but different points.
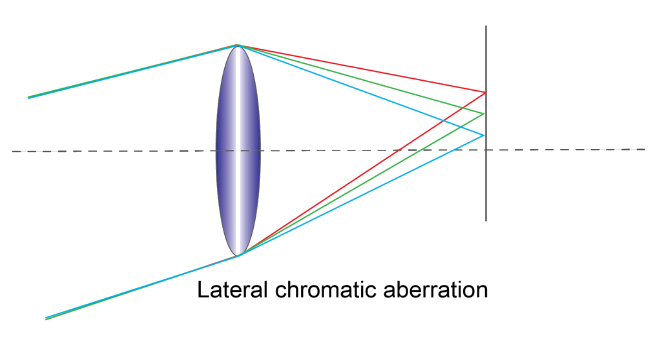
2. Longitudinal chromatic aberration: In this aberration, the light rays of different wavelengths concentrate at different points along the principal axis (horizontal line) of the lens.
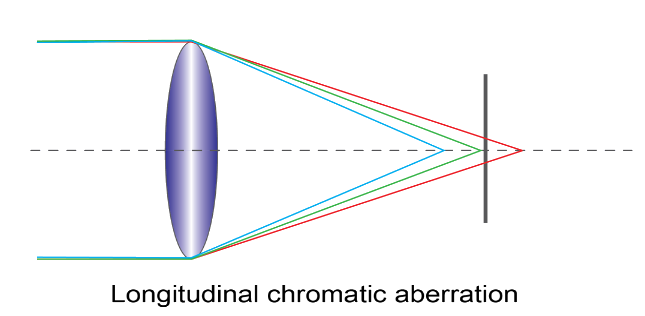
Correction of the chromatic aberration:
- Lateral chromatic aberration gets reduced when the object is placed at infinity.
- The combination of two lenses also reduces chromatic aberration.
- Achromatic lenses can be used to reduce chromatic aberration.
Axial color
Axial color is a phenomenon of aberration in which the position of the image shifts as the light wavelengths differ. Color fringing occurs due to color aberration. This aberration is caused by the light rays having different wavelengths to focus at various points.
Summary
For non-paraxial rays, the actual image differs from the result of the paraxial approximation. This defect of the image is known as an aberration. For monochromatic light or single-wavelength light, there are five types of aberration. It is impossible to eliminate all kinds of aberrations by using a single lens. We can use different lens combinations to eliminate them.
Frequently Asked Questions
Q1. What is a spherical aberration?
Ans: The spherical aberration is the defect of the image in which central and peripheral incident rays from images at different points on the axis, are known as spherical aberration.
Q2. How to correct the coma aberration?
Ans: Coma can be corrected by bending the lens. This process will only work in the case of a single lens.
Q3. What is the one difference between chromatic aberration and spherical aberration?
Ans: Chromatic aberration is shown by white-colored light but the spherical aberration is shown by monochromatic lights.
Q4. What is the principal axis?
Ans: The principal axis is the axis that passes through the focus and center of curvature of the lens.
Q5. What is a positive coma?
Ans: When the lateral magnification of the outer zone is greater than the central zone, then the coma is said to be a positive coma.
Q6: White light is the polychromatic source of light. Explain why?
Ans: white light is the polychromatic source of light because it constitutes seven colors and each of them has a different wavelength.