Introduction
Proteins play a crucial role in muscle movement. They form the building blocks of muscle fibers and are involved in all aspects of muscle function, including muscle contraction, regulation of muscle tone, and maintenance of muscle structure. The interaction between contractile, regulatory, and structural proteins, as well as enzymes involved in energy metabolism, is necessary for the proper functioning of muscles and the production of movement.
What are muscle proteins?
Muscles are tissues in the body that are specialized for movement and are essential for many bodily functions, including posture, locomotion, and maintaining stability. They are composed of muscle fibers, which are long, cylindrical cells that contract and relax to move. The contractile activity of muscle fibers is enabled by various muscle proteins, which work together to generate force and produce movement.
The muscle protein can be classified into,
- Myofibrillar proteins
- Regulatory proteins
- contractile proteins
- Stromal proteins
- Structural proteins
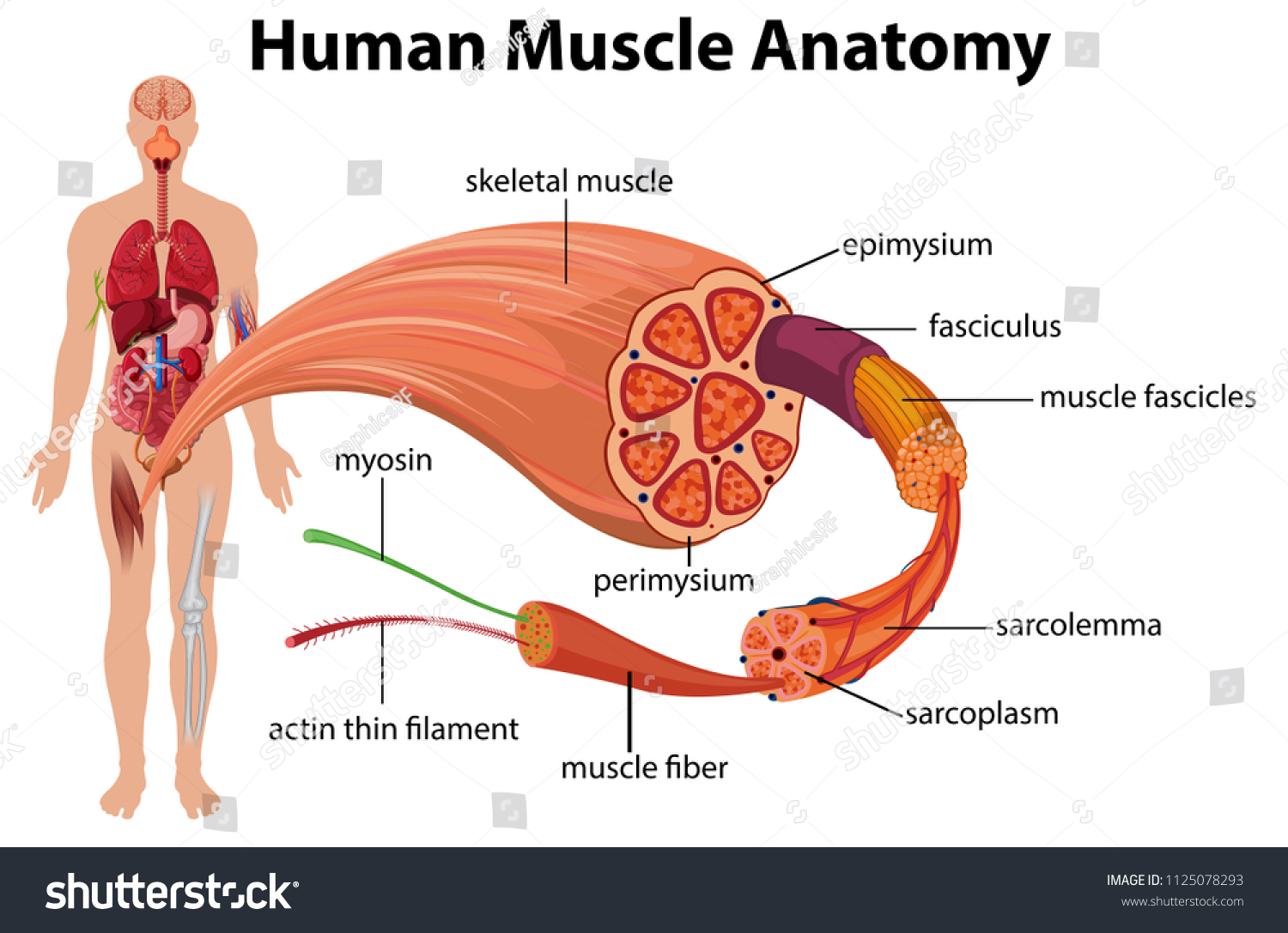
Myofibrillar proteins
Myofibrillar proteins are a group of contractile proteins that are found within muscle fibers and are essential for muscle contraction. They are organized into repeating structural units called sarcomeres, the basic contractile muscle units.
- The most essential myofibrillar proteins are actin and myosin, which form the thin and thick filaments, respectively, within each sarcomere.
- Actin filaments are composed of actin subunits that are organized into a double helix structure, while myosin filaments are composed of myosin protein molecules that are organized into long, rod-like structures.
- During muscle contraction, The sarcomere shortens and the muscle fiber contracts as the actin and myosin filaments slip past one another.
- This process is regulated by other myofibrillar proteins, such as troponin and tropomyosin, which control the interaction between actin and myosin and help to coordinate muscle contraction.
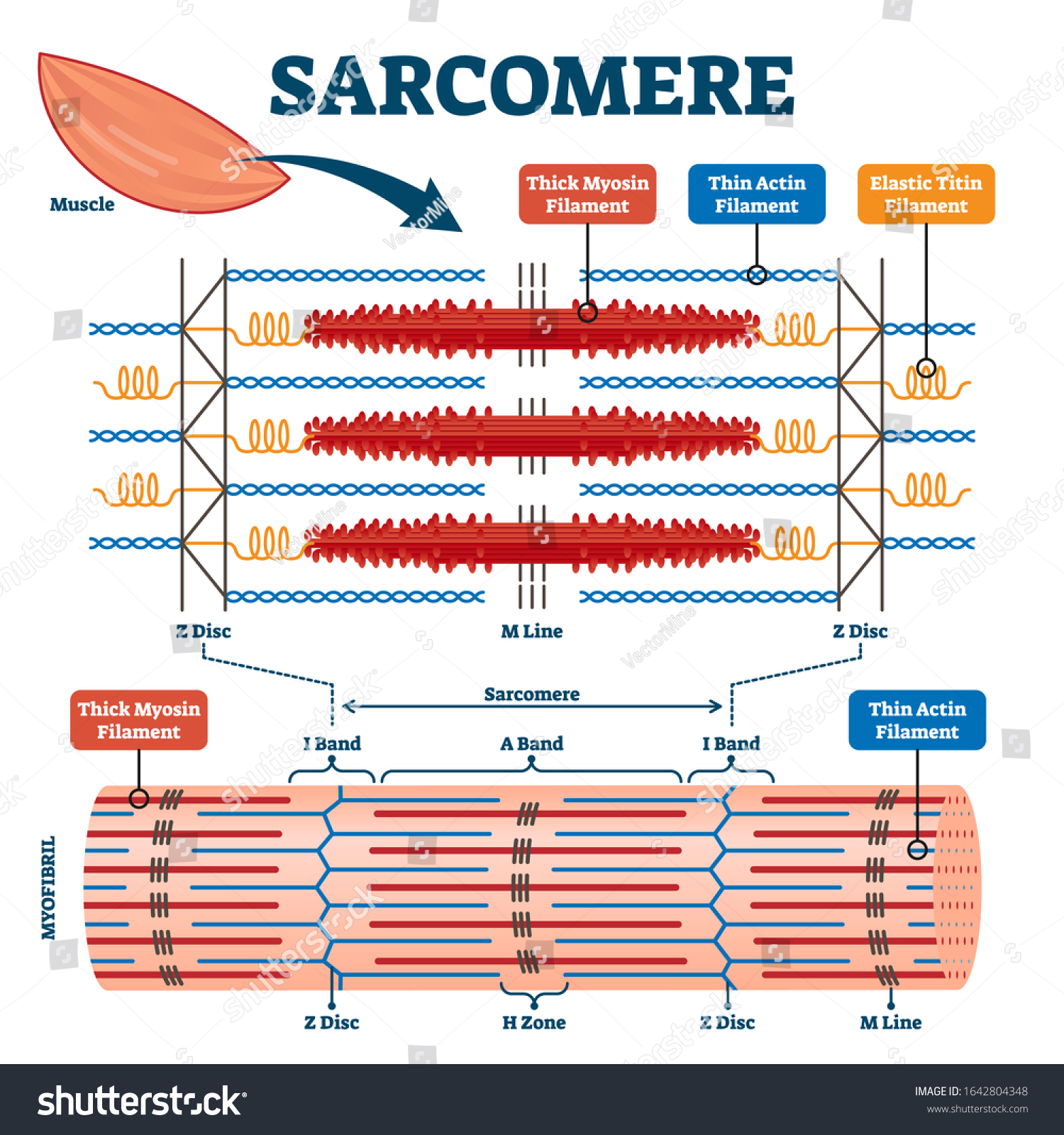
Contractile proteins
Contractile proteins are the proteins that are directly involved in the contraction of muscle fibers. They are essential for enabling movement, posture, and other physiological processes that require muscle contraction.
- Actin and myosin are the two most significant contractile proteins in muscles. Within muscle fibers, myosin creates thick filaments, whereas actin creates thin filaments. During muscle contraction, these filaments slide past each other, producing a shortening of the muscle fiber.
- Another important contractile protein is troponin, which is part of a complex of proteins that regulates the interaction between actin and myosin. Troponin binds to calcium ions, which are necessary for muscle contraction, and helps to control the interaction between actin and myosin.
- Tropomyosin is another protein that plays a role in muscle contraction. It runs along the actin filaments and helps regulate the interaction between actin and myosin.
- Titin is a large protein that helps maintain the structure of muscle fibers and plays a role in the elasticity of muscle.
Regulatory proteins
These proteins help in regulating the activity of other proteins in muscle fibers, including contractile and metabolic proteins. They help to coordinate muscle contraction and maintain the proper functioning of muscle fibers.
Some examples of regulatory proteins in muscle include:
- Calcium-binding proteins: Calcium ions are essential for muscle contraction, and calcium-binding proteins help to regulate the concentration of calcium ions in muscle fibers. One important calcium-binding protein is calmodulin, which helps to control the interaction between actin and myosin.
- Enzymes involved in energy metabolism: These enzymes help to regulate the production and utilization of energy in muscle fibers and include creatine kinase, lactate dehydrogenase, and others.
Structural proteins in muscle
Structural proteins are proteins that help maintain the structural integrity of muscle fibers and provide support to the muscle. They play a role in maintaining the shape and stability of muscle fibers, as well as enabling proper muscle function.
Some examples of structural proteins in muscle include:
- Titin: Titin is a large protein that helps maintain the structural integrity of muscle fibers and plays a role in the elasticity of muscle.
- Dystrophin: An essential component of healthy muscular function is the protein dystrophin, which aids in preserving the structural stability of muscle fibers. Mutations in the dystrophin gene are responsible for a form of muscular dystrophy called Duchenne muscular dystrophy.
Stromal proteins
Stromal proteins are proteins that are found in the extracellular matrix (ECM) of muscle fibers and play a role in maintaining the structural integrity and proper function of muscle. The ECM is a complex network of proteins and carbohydrates that provides support to the cells and tissues of the body.
Some examples of stromal proteins in muscle include:
- Collagen: Collagen is a fibrous protein that provides structural support to muscle fibers and helps to maintain the strength and stability of muscle tissue.
- Elastin: Elastin is a protein that gives muscle fibers elasticity, allowing them to stretch and then return to their original shape.
- Fibronectin: Fibronectin is a protein that helps to connect muscle fibers to other tissues and provides support to the muscle.
Summary
Muscle contraction is the process by which muscle fibers shorten, generating force and movement. Actin and myosin are the primary contractile proteins in muscle fibers. They work together to create the dense filaments that makeup muscle fibers. When muscles are contracting, the myosin filaments interact with the actin filaments and cause them to slide past each other, producing a shortening of the muscle fiber. Regulatory proteins, such as troponin and tropomyosin, help to coordinate the interaction between actin and myosin and regulate the initiation of muscle contraction. Structural proteins, such as titin, provide support to the muscle and help maintain the structural integrity of muscle fibers.
Frequently Asked Questions
1. What are the 3 roles of ATP in muscle contraction?
It also serves as a reminder that ATP is necessary for the muscle cell’s ability to energize the myosin cross bridge, detach the cross bridge from the actin-binding site, and transport calcium ions back into the SR.
2. What is troponin in muscle contraction?
Troponin is the sarcomeric Ca2+ regulator for striated (skeletal and cardiac) muscle contraction (Tn). When Tn binds Ca2+ and undergoes structural changes in the actin-tropomyosin filaments, myosin ATPase activity and muscle contraction are triggered.
3. Role of Calcium ions in muscle contraction.
Calcium ions are crucial for muscle contraction because they help myosin and actin interact with one another. To promote muscular contraction, the myosin head is linked to the binding region that is exposed when the Ca2+ ions bind to the C part of the actin filament.