Introduction
Organic reactions discovered by a specific scientist are known as “named reactions,” and the namesake scientist is often credited with the discovery. Many of these reactions have important practical and economic applications. One such reaction with a name in organic chemistry is the Finkelstein reaction. Hans Finkelstein, a German chemist, first described what is now known as the Finkelstein reaction. Because of its significance, this organic reaction bears the scientist’s name. This. Organic exchange reactions include the swapping of one halogen atom from one chemical for another halogen atom from another.
What is the Finkelstein Reaction?
Finkelstein reaction follows \(S{N_2}\) mechanism which involves the replacement of halogen atom. The reaction leads to the formation of alkyl iodide by the reaction of alkyl halides with metal halide in the presence of a polar aprotic solvent. The reaction takes place as follows:
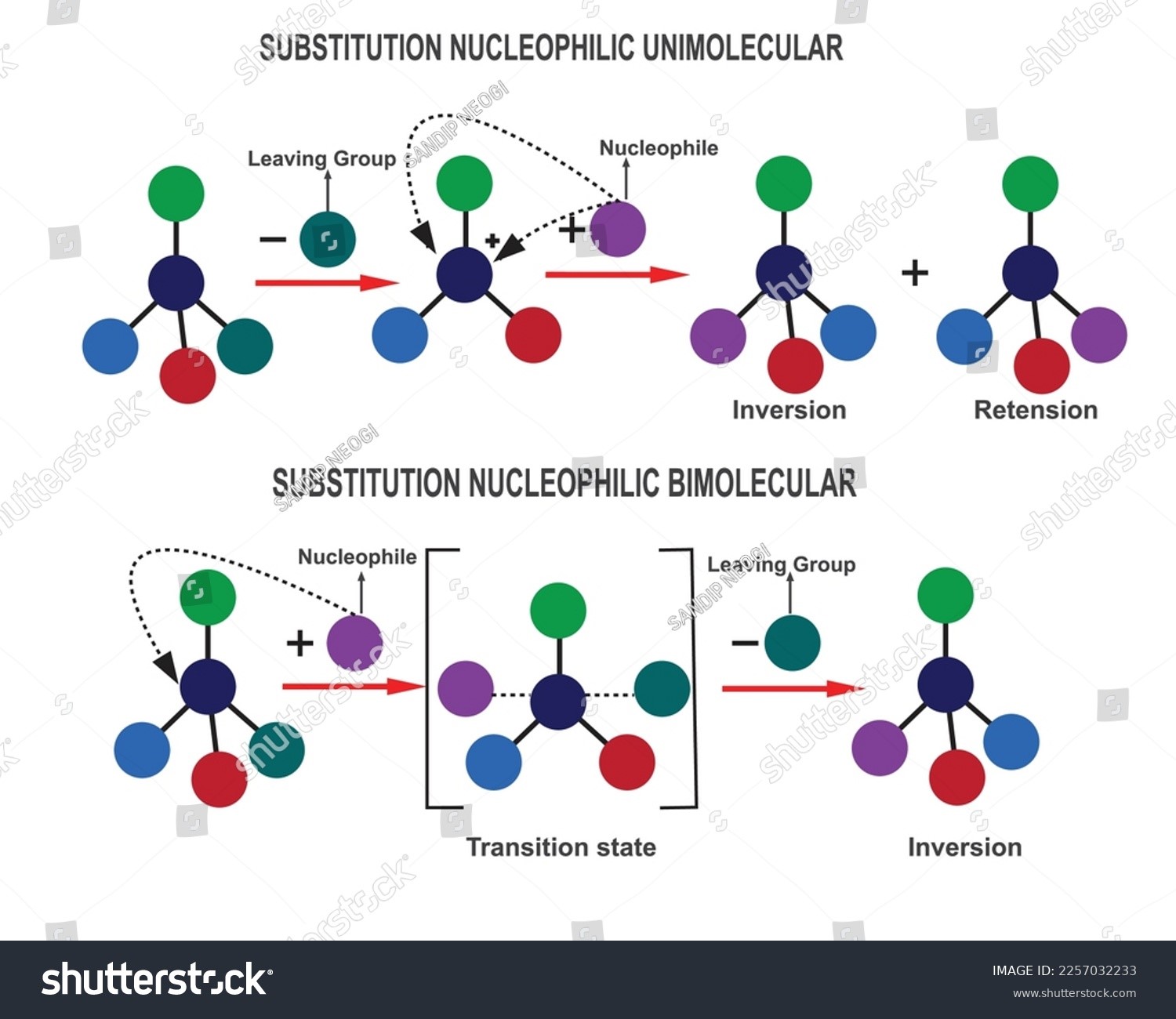
The alkyl halide should be primary. The halogens used for this reaction may be Chlorine or Bromine. The solvent used for the reaction is acetic acid which is a polar aprotic solvent.
Examples of Finkelstein Reaction
Many alkyl halides can be made or synthesized with the aid of Finkelstein’s reaction, some examples are:
- The reaction of sodium iodide with methyl bromide results in the formation of methyl iodide is an example of Finkelstein’s reaction. And sodium bromide is obtained as a by-product. The reaction is:

- The reaction of sodium iodide with ethyl chloride results in the formation of ethyl iodide is also an example of Finkelstein’s reaction. And sodium chloride is obtained as an acid by-product. The reaction is,

- The reaction of Sodium iodide with ethyl bromide results in the formation of ethyl iodide is also an example of Finkelstein’s reaction. And sodium bromide is obtained acid by-product. The reaction is,
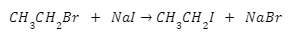
Finkelstein Reaction Mechanism
- The reaction process is straightforward and simple to grasp because it follows an SN2 mechanism.
- The stereochemistry of the reactant is flipped at the end of the reaction.
- The forward reaction is caused by metal halides’ low solubility.
- The alkyl halides are attacked by the nucleophile in the sodium iodide in a single step reaction.
- Which then leads to the production of alkyl iodide and sodium halide.
- The sodium bromide or sodium chloride precipitates out of solution during the process but is miscible with acetone.
Aromatic Finkelstein Reaction
A catalyst, in addition to the Finkelstein reactants, can increase the efficiency of the Aromatic Finkelstein Reaction.
Aromatic Finkelstein reaction catalysed by copper(I) iodide in the presence of diamine ligands. Tri-n-butyl phosphine and Nickel bromide are two other catalysts that can be used. As shown in the procedure below, Benzyl iodide can be synthesised from benzyl chloride by reacting sodium iodide with acetone.
The oxidative addition reaction is the first stage of the aromatic Finkelstein reaction using a copper catalyst. After that, halide exchanges occur, and finally, reductive elimination takes place, which ultimately leads to the regeneration of an analogous catalyst. An excellent catalyst for the aromatic Finkelstein reaction is Copper (I) Iodide in the presence of a diamine ligand. The following illustration depicts a copper-catalyzed reaction between aromatic halides.
Uses of Finkelstein Reaction
- This reaction is widely employed for the production of alkyl iodides for application in industry.
- In addition, it is employed in the analysis of a specific group of alkyl iodides.
- It is through this process that chrysochlamic acid is generated.
- It is a necessary step in the production of -iodoalkyl esters.
Summary
Finkelstein reaction is an organic named reaction that involves the production of alkyl iodide in a better way. The production of alkyl iodides is a complex mechanism, but it is made easy with the help of this reaction. It involves the use of alkyl halides and metal iodide such as sodium iodide. An organic polar aprotic solvent, acetone is used in the reaction. It is a single step by the molecular reaction which follows the \(S{N_2}\ mechanism. The first step in the reaction is the nucleophilic attack. And then the corresponding replacement of halogens takes place and alkyl iodides will be formed. The sodium salt is also precipitated in the reaction. The reaction also has some other applications, especially in the analysis of alkyl halides.
Frequently Asked Questions
1. What is the significance of NaI in Finkelstein’s reaction?
Ans. Sodium iodide has high covalent character than other sodium halides and thus it makes it efficient than other halides as well as other iodine compounds.
2. What are the limitations of the Finkelstein Reaction?
Ans: The limitations of the Finkelstein Reaction include the fact that it is not applicable to tertiary alkyl halides, and that it is not applicable to aryl halides.
3. Why is Finkelstein reaction reversible?
Ans. The reaction described by Finkelstein illustrates the exchange of one halogen for another. Since various metal halide salts are soluble in acetone at varying concentrations, halide exchange can occur in both directions.