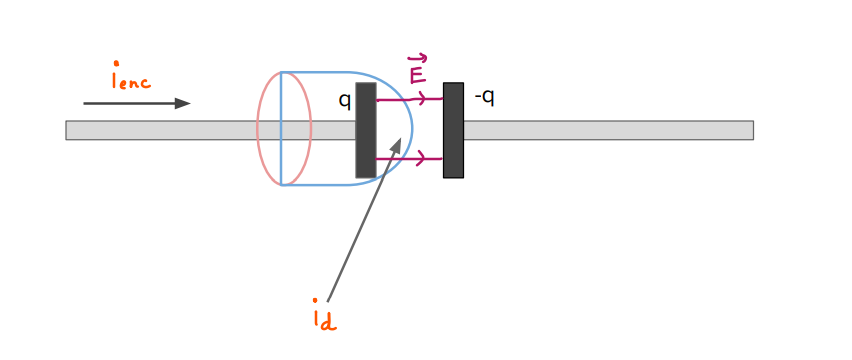
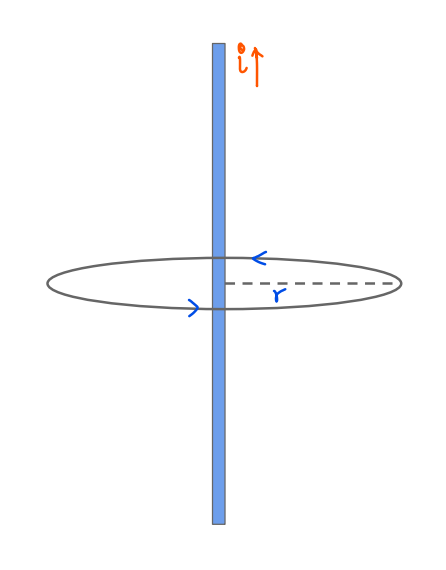
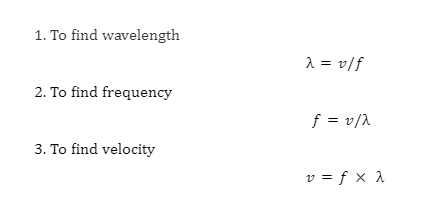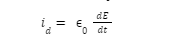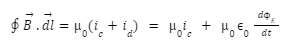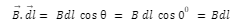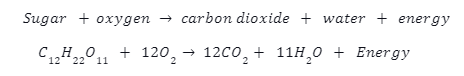Introduction
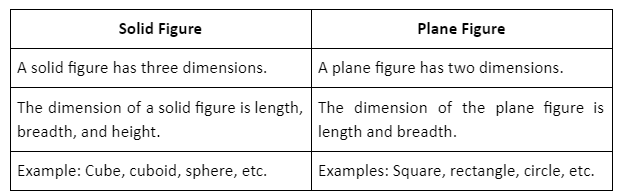
A plane slanted at a particular angle is known as an inclined plane. Such a plane is connected to the ground on one end while the other end is raised by a given height that is referred to as the slant height.
The angle of inclination for an inclined plane is defined as that angle at which an object just starts to slide down due to its own weight.
 Inclined plane AB
Inclined plane AB
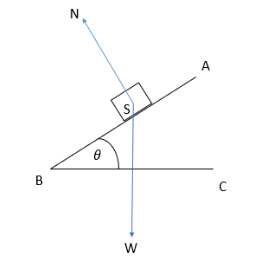
Body on an inclined plane
Normal force in inclined planes
Generally, it is observed that the normal reaction due to gravitational forces acts just opposite to the weight of the body, especially when placed on the ground. However, that is not universally true. In fact, the normal reaction in the case of an inclined plane acts in a direction that is perpendicular to the incline, not the ground.
Frictional force
Friction occurs when two surfaces come into contact and its tendency is to resist motion. Thus, it always acts opposite to the direction of motion of an object. For an object sliding down an inclined plane, frictional force acts up the incline and vice versa.
Gravitational force components
The gravitational force may be resolved into two components when dealing with an inclined plane.
- The normal/vertical part acts perpendicularly to the incline and downwards from it. In the diagram below, it is represented as \({W_{V}}\).
- Similarly, there exists a horizontal component of the body’s weight that acts parallel to the incline and points downwards. It is represented by \({W_{H}}\)in the diagram.
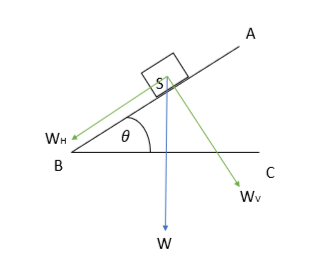
Resolution of gravitational force
The body is at equilibrium
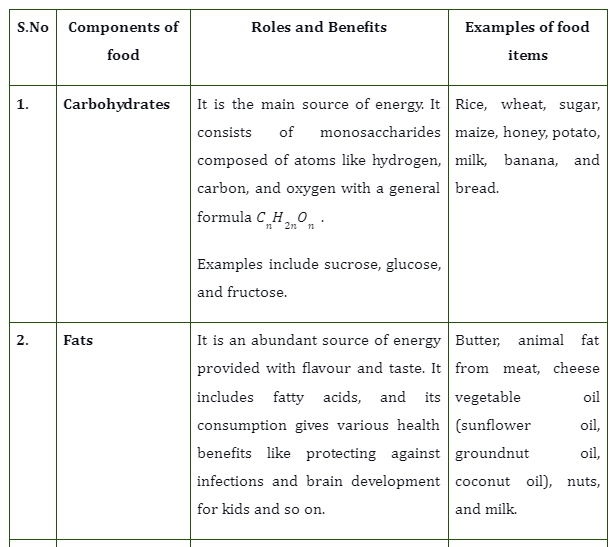
In the diagram,
N= normal force
W= weight of the body (gravitational force)
\({W_{H}}\)=Horizontal component of the gravitational force
\({W_{V}}\)= Vertical component of the gravitational force
f= frictional force
And we are given that the body is at equilibrium. In such a case,
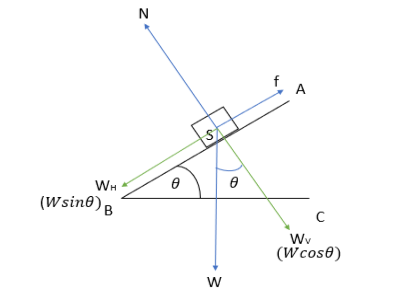
Body in equilibrium at incline
We can equate the horizontal and vertical forces. Let us start with the horizontal ones:
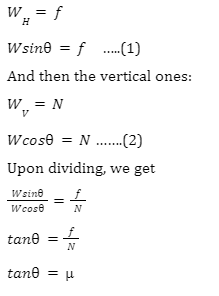
The quantity μ is known as the coefficient of friction.
Equilibrium of a body on an incline plane:
Two limiting cases of equilibrium exist on an inclined plane:
- The body is moving up the incline or,
- It is falling down the incline.
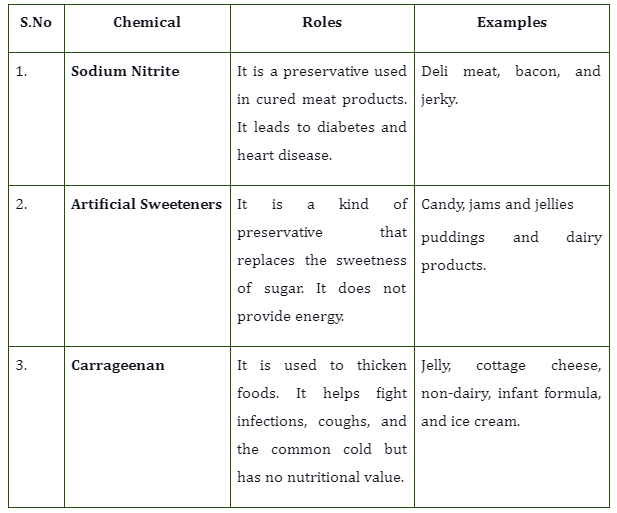
A body moving down the incline.
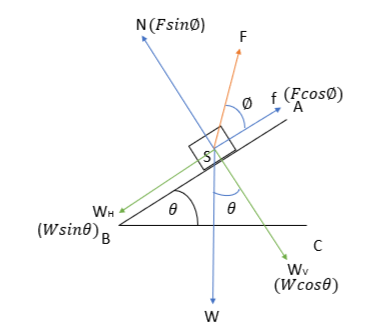
When the body moves down the incline, we have
N= normal force
W= weight of the body (gravitational force)
\({W_{H}}\)=Horizontal component of the gravitational force
\({W_{V}}\)= Vertical component of the gravitational force
f= frictional force
F= external force
Applying the conditions of equilibrium,

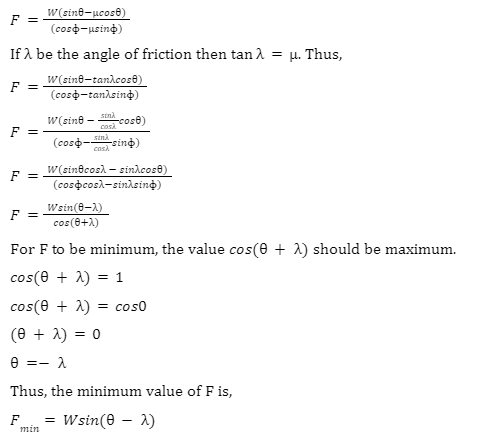
A body moving down the incline.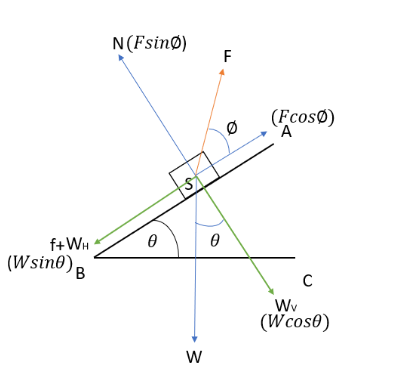
As before,
N= normal force
W= weight of the body (gravitational force)
\({W_{H}}\)=Horizontal component of the gravitational force
\({W_{V}}\)= Vertical component of the gravitational force
f= frictional force
F=external force
We proceed just as in the previous example.

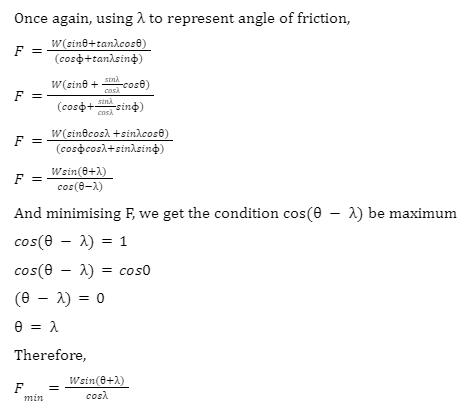
Solved problems on inclined plane
1. If a block kept on an inclined plane will start slipping, find the angle of inclination given that the coefficient of static friction = 1.73.
We are given the value of = 1.73. We can use the fact that
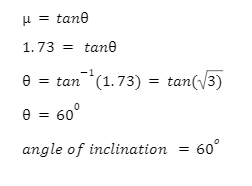
2. If a block of mass 10 kg remains at rest on an incline of\({45^{0}}\), find the force of friction. (Take\(g=10 m{s^{ – 1}}\))
Given that, the block is at rest so that net force parallel to the incline is zero.
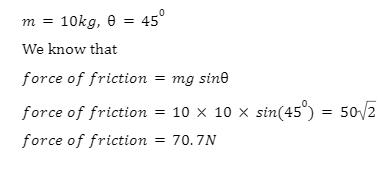
Summary
An inclined plane is a sloping surface designed to move heavy machinery and tools that cannot be lifted directly. Various types of inclined surfaces such as ramps, stairs, and slides exist.
The primary benefit of an inclined plane is that it helps us move large objects by applying a lesser amount of force. The angle of inclination plays a critical role in determining how much force we must apply and if we would be able to move the object in question at all. For the object to not fall down back on us, we must move it up the incline at a certain angle of friction.
Frequently Asked Questions
1. How many components of gravitational force do we have on an inclined plane?
There are two components we can resolve the gravitational force into:
- Horizontal component and,
- Vertical component.
2. Write down two functions of an inclined plane?
Inclined plane has a large number of uses, with the primary ones being,
- They function as simple machines.
- They help us move heavy objects upward with ease.
3. What are the forces experienced by an object moving on an inclined plane?
The object experiences gravitational force, normal reaction, and frictional force.
4. In what direction do frictional forces act?
Frictional forces always act opposite the direction of motion of an object.
5. What is the angle of inclination?
It is that angle at which, when a body is placed on an inclined plane, it will start sliding down due to its own weight.

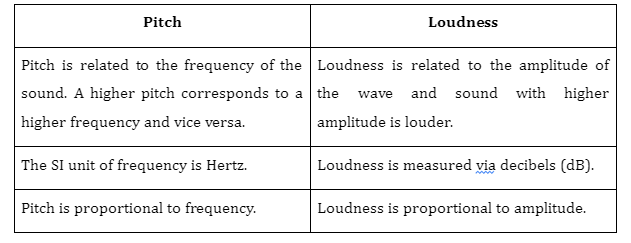 2. Are pitch and frequency different?
2. Are pitch and frequency different?