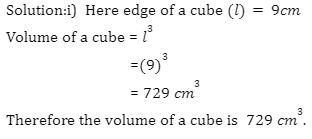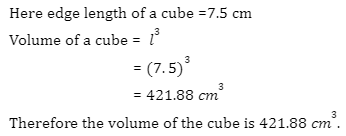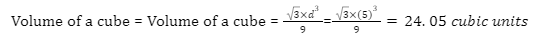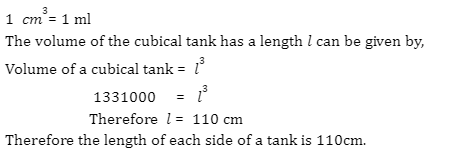Introduction
A battery is an electronic device that converts chemical energy to electrical energy through a redox reaction. They are typically composed of a combination of cells, with cells being the fundamental unit of batteries. Energy is stored in these cells and drawn as current and thus, the output of any battery depends on the combination of cells used inside it.
There are two terminals on batteries, of which, one end is known as the cathode and the other end as the anode. Upon connecting a circuit to the battery, electrons can move from one node to the other and thus, current can flow through the circuit. In electrical circuits, batteries and cells can be connected in various combinations such as series, parallel, and even mixed. Today, various types of batteries are available in the market, including Pb-acid batteries, Li-ion batteries, Ni-Cd batteries, etc.
Important terms related to battery
EMF of battery: When no external factors and voltage sources are present, the potential difference between the two terminals of the battery is known as its emf, short for electromotive force.
Terminal voltage: When current starts flowing in the circuit, the potential difference between the battery’s terminals is termed as terminal voltage. Note that since the battery is an electrical device, resistance to current flow is natural. The resistance offered by the battery itself is known as internal resistance, and it creates a voltage drop across its terminals.
Let V be this voltage drop and E be the emf of the battery. Then,
Internal voltage drop= E-V
Internal resistance: As mentioned, the battery has an inherent resistance inside it, which is known as internal resistance.
Batteries in series and parallel
Series combination
This is like creating a train of batteries. The positive terminal of each battery is connected to the negative terminal of the next one and in this arrangement, the same amount of current flows through each of them. Hence, the voltages offered by the batteries can be added algebraically to get:
![]()
Parallel combination
In this combination, the positive terminals of all the batteries are connected to each other and the same is done for all the negative ones. In this configuration, the voltages dropped across the terminals remain the same and we can add current algebraically.
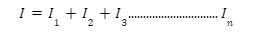
Connecting batteries in series
Series combination is used when we need to increase the voltage without changing the amount of current. Here is an example:
Say we had a battery of EMF 20 V and capacity 100 A-h. Then, if we connect them in series as shown below, we would end up with a combination that yielded us 40 V output but with no change in capacity.
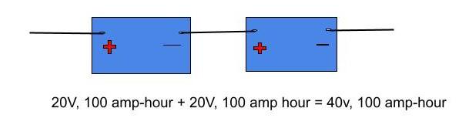
Series combination
Hence, we get an output with increased voltage. Note that the capacities of the batteries must be the same or they might get damaged.
Advantages of Series Combination
- Since the current flow does not increase, there is no additional heat generated.
- It is simpler than parallel combinations.
- The voltage drop increases.
Disadvantages of Series Combination
- Any break or damage in the circuit at any point will damage the circuit.
- Increased number of components also increases the resistance of the circuit.
- A low voltage system is not compatible with this combination.
Batteries in Parallel
If we wish to increase the capacities of batteries without changing the output voltage, we use a parallel configuration. This increases the current flow in the circuit. For instance, given two batteries of 24 V, 100 A-h each, connecting them in parallel would give us a battery that provides 200 A-h at 24 V.
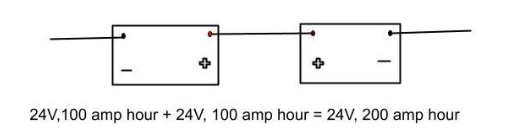
Parallel Combination
This sort of combination is used in solar panels.
Advantages of Parallel Combination
- The capacity increases, yielding to increase in use-time,
- The voltage remains the same.
Disadvantages of Parallel Combination
- The current is increased, causing heat to increase.
- Charging batteries in this configuration takes longer.
Mixed Combination
As implied by the name, mixed combination is the combination of some batteries connected in series linked together in parallel combination or vice versa. This is a complex arrangement that can help us generate almost any combination we want from a given bunch of batteries.
For example, suppose we were given six batteries of 15 V and 100 A-h each. We could combine them as follows:
- Set 1 – B1 and B2 linked in series = 15V + 15V = 30V, 100 amp hour
- Set 2 – B3 and B4 linked in series = 15V +15V = 30V, 100 amp hour
- Set 3 – B5 and B6 linked in series = 15V + 15V = 30V, 100 amp hour
Next, we would combine the above sets in parallel, giving us a 30 V, 300 A-h configuration, visible in the diagram below.
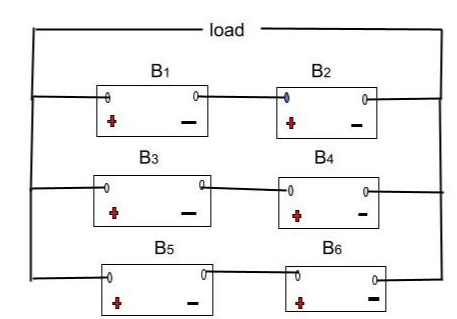
Mixed combination
Hence, the effective voltage drop will remain at 30 V, but the capacity would be three-folds.
Summary
Depending on our requirements and the scenario in question, different combinations of batteries can be used. The most commonly used ones are series, parallel, and mixed grouping of batteries. What we use is entirely up to us and depends on the requirements of the circuit. The output of the configuration depends on its design and thus, if we needed higher voltage, we would opt for series combination and if needed higher capacity, we would use parallel configuration. Similarly, mixed grouping allows us to generate arbitrary configurations from a given set of batteries.
Frequently Asked Questions
1. Can we put a 12V 100 amp hour battery and a 15V 200 amp hour battery in a series combination?
No, since they have different capacities. For series combination, the capacities of the batteries must be the same.
2. How does a battery work?
A battery is composed of electrochemical cells, which convert chemical energy into electrical energy. When connected to a circuit, ions flow inside, leading to the flow of current.
3. Name the reaction that takes place in the batteries
The process is known as redox reaction and it involves reduction and oxidation simultaneously.
4. How are batteries classified?
Batteries may be classified as follows:
- Primary batteries: These are one-time batteries which can not be recharged after they get depleted.
- Secondary batteries: These batteries can be recharged after use, giving them longer life.
5. What type of electrical combination is used for domestic purposes and why?
In domestic applications, parallel combination is used since it supplies an equal amount of voltage to all the devices, which is necessary for proper functioning.