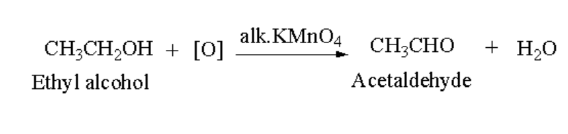
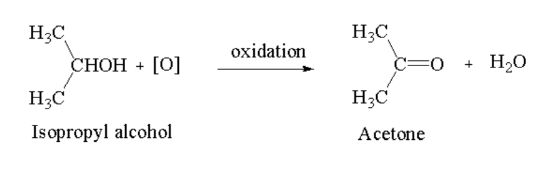
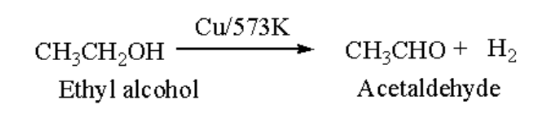
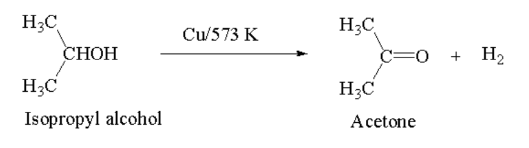
Introduction
Shakespeare is universally regarded as the greatest English language author and the pinnacle of dramatic art. The “Bard of Avon” and “England’s National Poet” are two of his other nicknames. Around 38 plays, 154 sonnets, two substantial narrative poems, and a few more verses, the authorship of which is disputed by some, are attributed to him.

William Shakespeare
His Life, Career and Marriage.
The term “The Bard” is commonly used to refer to William Shakespeare. Throughout the Elizabethan era, Shakespeare penned a number of plays that have become literary masterpieces and are still performed and studied today. Many believe him to be among the finest speakers of the English language due to his ability to create new words, understand humour, and masterfully employ sarcasm and puns.
Shakespeare wed Anne Hathaway at age 18 while they were both very young. She was eight years older than him at the time. They had three children: the identical twins Hamnet and Judith, and Susanna. His life’s story grew even more mysterious following his marriage. It is believed that he spent the most of his time in London, where he wrote and directed his plays. He started a successful acting career in London between the years 1585 and 1592. Formerly known as the Lord Chamberlain’s Men, he eventually became the group’s writer and co-owner under the stage name the King’s Men.
Death and retirement
Aged 59 after shifting to Stratford, where he retired around 1613, and died there three years later. Shakespeare’s personal life is not well documented. On April 23, 1616, he passed away at the age of 52. He signed his will and declared that he was in “great health” at the time, but he died a month later..
His Career
Due to the lack of precise records being kept in the 1500s moreover William Shakespeare wasn’t as well-known back then as he is now, therefore earlier material is less accessible.
Shakespeare’s most well-known works were created in 1589 and 1613. His early pieces, which were primarily comedies and histories, are still recognised as some of the best in their respective categories. Up until around 1608, most of his works were tragedies, among them Hamlet, Othello, King Lear, and Macbeth, which are regarded as some of the best in the English language. In his final period, he cooperated with other playwrights and composed tragicomedies, also referred to as romances.
According to several academics, Hamnet’s passing was a pivotal moment for Shakespeare and served as the model for the character of Hamlet in his well-known play.
Works by Shakespeare: Plays
William Shakespeare moved to London not long after the birth of his children, and he quickly rose to prominence there as a playwright within the span of just seven years. Even after the group changed its name to The Kings Men, Shakespeare maintained his loyalty to them throughout his career.
The original location for staging Shakespeare’s plays was the James Burbage Theater. At the original Theatre, the seats were on the stage. After losing the Theatre’s lease, Burbage asked Shakespeare and his company to help fund his next construction endeavour, The Globe.
The Globe, an outdoor theatre with a circular design and many levels of seats, regularly staged performances of Shakespeare’s plays. In the cheapest section, which had no seats, prices were very low. The wealthy and royalty sat on the third and second levels to see shows. The performance at the Globe was special since it made use of natural light and had fewer sets than usual. No one was wounded when a fire broke out during a performance of Henry VIII at the Globe Theater in 1613. The next year, construction began on the new Globe. The original Globe was destroyed in 1644, but a 1997 copy is still welcoming visitors today.
Conclusion
Shakespeare’s plays are still widely read today and are frequently performed, researched, and reinterpreted in many social, cultural, and political contexts around the globe. Shakespeare bequeathed his eldest daughter Susanna the majority of his sizable assets in his testament.
Frequently Asked Questions
1. What is regarded as William Shakespeare’s life’s work?
Ans. From there on, up until 1608, Shakespeare primarily produced tragedies, some of the best in the English language being Romeo and Juliet, Othello, Hamlet, King Lear, and Macbeth. In the latter part of his life, he cooperated with other authors and penned tragicomedies, often known as romances.
2 How was Shakespeare related tp Globe theatre?
Ans. He started writing plays and performing for the Lord Chamberlain’s Men in 1594. When James I became its patron, the group’s name was changed to the King’s Men, and he eventually became its resident playwright. In 1599, he co founded the renowned Globe theatre with other members.
3. Why is Shakespeare’s writing significant?
Ans. Shakespeare’s writing style developed independently of the prevailing fashion at the time. Shakespeare used an extremely stylized kind of metre called iambic pentameter, which has ten syllables each phrase with each unstressed syllable being followed by a stressed syllable.