Introduction
The electronic configuration characterises the distribution of electrons inside a subshell of an atom. An electron configuration is a schematic depiction of the predicted location of electrons in an orbit around a nucleus. In a neutral atom, the number of electrons equals the number of protons. We can visualise the electrons’ energy and the sort of orbital in which they are located by arranging them to stand around the nucleus. Electrons inhabit certain orbitals in a specific order depending on their energy.
What is Electronic Configuration
- The electronic configuration characterises the distribution of electrons inside a subshell of an atom.
- There is a consistent format for listing the subshells of an atom that contain electrons in an atomic electronic configuration.
- When dealing with many atoms, the conventional depiction of electrical configuration might become tedious. Sometimes, a shortened or abbreviated sign can be used for the full one.
- For instance, the Cl atom has an electron configuration of \({\bf{1}}{{\bf{s}}^2}{\bf{2}}{{\bf{s}}^2}{\bf{2}}{{\bf{p}}^6}{\bf{3}}{{\bf{s}}^2}{\bf{3}}{{\bf{p}}^6}\).
Subshells
- The distribution of electrons into subshells is determined by the azimuthal quantum no., symbolised by the letter “l.”
- The magnitude of the principal quantum no., n, dictates the magnitude of this quantum number. As a result, four distinct subshells can exist when n is equal to 4.
- The s, p, d, & f subshells, accordingly, relate to l=0,1,2, 3 quantities for n = 4.
- The highest electrons no. that a subshell may hold is given by the equation 2(2l+1)
- The greatest number of electrons that the s, p, d, & f subshells can accommodate is 2, 6, 10, and 14 respectively.
Filling of Atomic Orbitals
The following concepts govern how the electrons in the atomic orbitals are occupied.
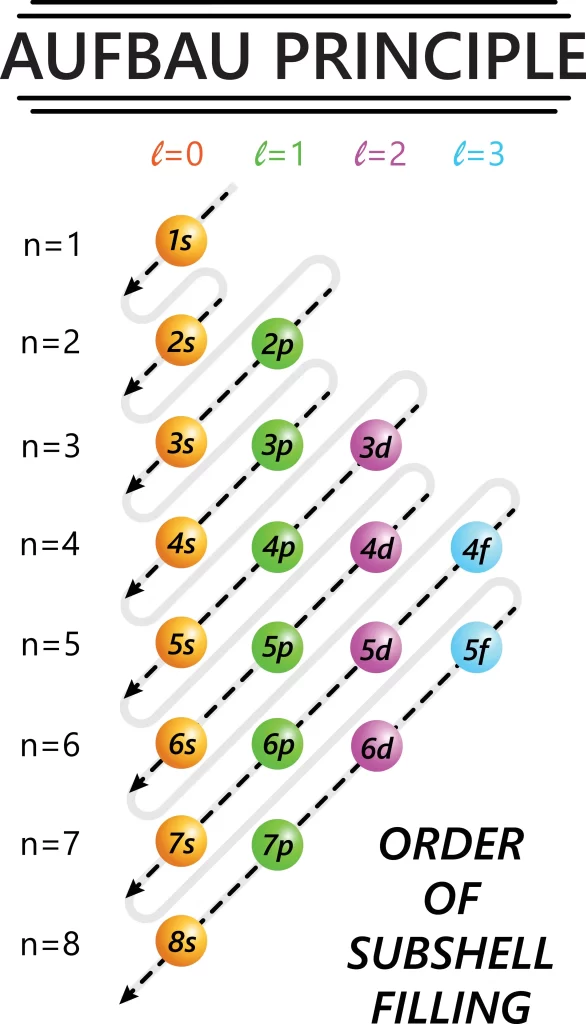
- Aufbau Principle; An atomic orbital’s energy is governed by its principal and azimuthal quantum number. The Aufbau principle says that electrons enter relatively low-energy orbitals and go to greater energy.
- Pauli Exclusion Principle: A maximum of two electrons of opposite spins can be carried in a subshell. This makes sure that every electron has a different set of quantum numbers.
- Hund’s rule; Each orbital in a particular subshell is said to be solely filled by electrons before a 2nd electron is placed in an orbital.
Aufbau Principle
Writing the Electronic Configuration,
The above-mentioned three main rules govern the creation of electronic configuration documents. The electrical configuration of each constituent is established under their supervision. The energy of an electron’s orbit around the nucleus may be calculated for certain distances, or “energy states.” The energy associated with a certain energy state increases as one moves further from the nucleus. Yet, it is difficult for everyone to retain the electron energy level diagram for numerous electron combinations.
The subshells are filled according to the Aufbau principle. The electrons occupy this sequence:
1s,2s,2p,3s,3p,4s,3d,4p,5s,4d,5p,4f,5d,6p,7s…
A maximum of two electrons can occupy a subshell.
Representation of Electronic Configuration of Atom
In this section, examples of a few elements’ electronic configurations are given:
- Helium:
Atomic number of Helium is 2. Its electronic configuration is \(1{s^2}\).
- Fluorine
The atomic no. of F is 9. Its electronic configuration is \({\bf{1}}{{\bf{s}}^2}{\bf{2}}{{\bf{s}}^2}{\bf{2}}{{\bf{p}}^6}\)
Summary
The electron configuration of an element represents the dispersion of electrons inside its atomic shells. As the electrons are mathematically positioned in these subshells, the configuration helps establish their location. The periodic table classifies elements into one of four groups based on the arrangements of their electrons. The s, p, d, and f blocks comprise these elements. How many electrons can fit inside a shell is proportional to the primary quantum number (n). The azimuthal quantum number (represented by the letter “l”) determines the subshell distribution of electrons.
Frequently Asked Questions
1. What are isoelectronic species?
Isoelectronic species are atoms or ions with the same number of electrons in their orbital. Thus isoelectronic species will have the same electronic configuration. However, this does not guarantee the same physical and chemical properties since the atomic numbers differ.
2. What is the importance of electronic configuration?
Electron configurations shed light on the chemical behaviour of an atom by revealing its valence electrons. It’s also useful for grouping things into categories like “s,” “p,” “d,” and “f” blocks which form the periodic table. It helps in accessing the similarity in properties of the elements.
3. Which subshells are present for n=1?
One orbital may contain a maximum of 2 electrons. For n=1, only the s subshell can exist. Its azimuthal number is 0. And only two electrons can be present in this subshell. Thus the possible configurations can be \(1{s^1}\) and \(1{s^2}\).