What are Aldehydes?
Carbon in an aldehyde forms double bonds to oxygen and single bonds to hydrogen and an alkyl group, making it a carbonyl functional group. The letter “R” stands for the alkyl group. Known as the carbonyl group, aldehyde chemical compounds all have a carbon atom double-bonded to oxygen. An aldehyde group’s carbon atom can come from any unsaturated, saturated, or cyclic carbon chain. A carbon atom in an aldehydic molecule will have at least one hydrogen atom attached to it.
Naturally Occurring Aldehydes
Naturally occurring aldehyde compounds are widespread in flora and fauna. Vanillin can be found in vanilla beans and cinnamaldehyde in cinnamon bark. Citral is extracted from lemongrass.
General Properties Of Aldehydes
Physical State: Aldehydes come in a wide range of physical forms. Formaldehyde is one such chemical that, at room temperature, exists in the gaseous state. Formalin is the name given to an aqueous solution of the substance. Most other common aldehydes are liquids at room temperature, however others, depending on the number of alkyl groups, are solids. The lower aldehydes have a strong odour, whereas the higher aldehydes are pleasant, which is why they are used in fragrances.
Boiling Points: Due to the dipolar attraction seen in the polar carbonyl group present in aldehyde, the boiling point of aldehyde is generally high.
Solubility: Water and other solvents are effective at dissolving aldehyde. The oxygen (O) in the C=O molecule has a lone pair of electrons, so it can form a H bond with the hydrogen in water. In general, aldehydes with four carbon atoms are soluble in water, although the solubility diminishes with increasing alkyl group size.
Uses Of Aldehydes
- Organic compounds like vanilla, orange rind, rose and citronella contain aldehyde. These compounds are created synthetically in laboratories and utilised for the production of perfumes and colognes due to their sweet smell.
- Aldehydes are used for making different organic compounds, dyes, and resins. Formaldehyde is prepared industrially on a larger scale and is used as a preservative for biological specimens and also has antibacterial properties.
Preparation of Aldehydes
There are many preparative methods for aldehydes. A few of them are discussed below.
Oxidation of primary alcohols: Primary alcohols can be treated with Pyridinium chlorochromate in dichloromethane to form aldehydes.
Dehydrogenation of primary alcohols : Dehydrogenation means the removal of the hydrogen molecule. Primary alcohol undergoes dehydrogenation in the availability of copper forms aldehyde.
From acid chlorides
The reduction of the acid chloride with hydrogen in the availability of palladium catalyst present over barium sulphate with a small quantity of quinoline gives aldehyde. The reaction is said as Rosenmund’s reaction.
Preparation from carboxylic acid
When the vapours of carboxylic acid are passed over manganese oxide at 573 K, the aldehyde is produced. A common example is passing formic through manganese oxide at 573 K to give formaldehyde, carbon dioxide and water.
Preparation from alkenes
Alkenes reacting with ozone in presence of carbon tetrachloride give ozonides which further break down into two molecules of aldehyde in presence of zinc dust and water.
Nomenclature of Aldehydes
Aldehydes can be identified with the suffix -al, in other words, any hydrocarbon name that ends with the suffix -al is referred to as aldehyde. For example methanal, ethanal, and propanal. The nomenclature of aldehyde is done using the IUPAC naming system. IUPAC stands for International union of pure and applied chemistry.
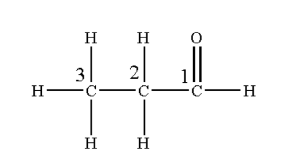
- First, the numbering of carbon atoms starts from the carbonyl carbon. Here, the number of total carbon (C) atoms is three so the hydrocarbon chain is “propane” and the root word is “propan”.
- As the aldehyde group is present (-CHO) the “ane” suffix is changed by “al” forming propanal.
- So, the IUPAC name of the given compound is propanal.
Structure of Aldehydes
The general structure of aldehyde is shown below.

Aldehyde
Here, the carbonyl carbon is \(s{p^{2}}\) hybridised. The chemical compounds containing carbonyl groups like aldehyde and ketone have planar geometry. The bond angle is 120 degrees. In the carbonyl group, the oxygen atom is more electronegative in nature than carbon which indicates that it is a polar group. As a result, the dipole moment is 2.3 D to 2.8 D.
Tests for Aldehydes
2, 4-DNP Test: Here, the organic compound is mixed with an acidic solution of 2, 4-dinitrophenylhydrazine along with methanol. After some time, a formation of a red precipitate is observed. The red precipitate is of 2,4-dinitrophenylhydrazone. The solid crystals are filtered and by recrystallisation method, solid mass is obtained. The hydrazone derivative has a distinct melting and boiling point which helps to detect the carbonyl group. Further, as the presence of the carbonyl group is detected, we can also find out whether the compound is ketone or aldehyde.
As the precipitate obtained is in solid form, it can be filtered out and the melting point of the solid can be determined. By comparing the melting point of the precipitate to the known value of ketone and aldehyde, the carbonyl group present in the compound can be detected.
Schiff’s reagent: It is a pink colour dye which gets decolourised by passing sulphur dioxide gas. When aldehyde is treated with Schiff’s reagent, the colour is retained.
Summary
Aldehyde is one of the classes of functional groups containing a carbonyl carbon attached to oxygen by a double bond and to an alkyl group and hydrogen by a single bond. The aldehyde can be identified by the suffix “al”. The general formula of aldehyde is R-CHO. Higher grade aldehydes are used for the preparation of perfumes and colouring agents due to their sweet smell. The aldehyde can also be synthesised by different methods as discussed. A few examples of aldehydes are formaldehyde, Ethanal, Propanal, Butanal etc. Some naturally occurring aldehydes are vanillin, citral, etc.
Frequently Asked Questions
1. Why are aromatic aldehydes less reactive than aliphatic aldehydes?
Ans: Aromatic aldehydes are less reactive because the lone pair of oxygen atoms is involved in resonance with the benzene molecule. This makes the carbonyl group less nucleophilic.
2. Which reducing agent is used for aldehydes?
Ans: Sodium Borohydride is also called as tetra hydrido borate is used as a reducing agent of aldehydes. The aldehydes are converted into primary alcohols.
3. Which type of aldehyde compound can undergo aldol condensation?
Ans: The aldehyde compound containing alpha hydrogen undergo aldol condensation. This is because only compounds with alpha hydrogen can form beta-hydroxy aldehydes, which is the end product of the reaction.