Introduction
Everything in the universe consists of tiny particles called molecules, which are classified based on the distance and the attractive force between their constituents. Different substances have distinct properties such as mass, temperature, density, volume, pressure, etc.
The process of transfer of heat is achieved through three different processes in matter, namely, conduction, convection, and radiation. It is studied under thermodynamics, which deals with thermal energy and its relationship to heat, work, temperature, energy, entropy, and other physical properties of matter.
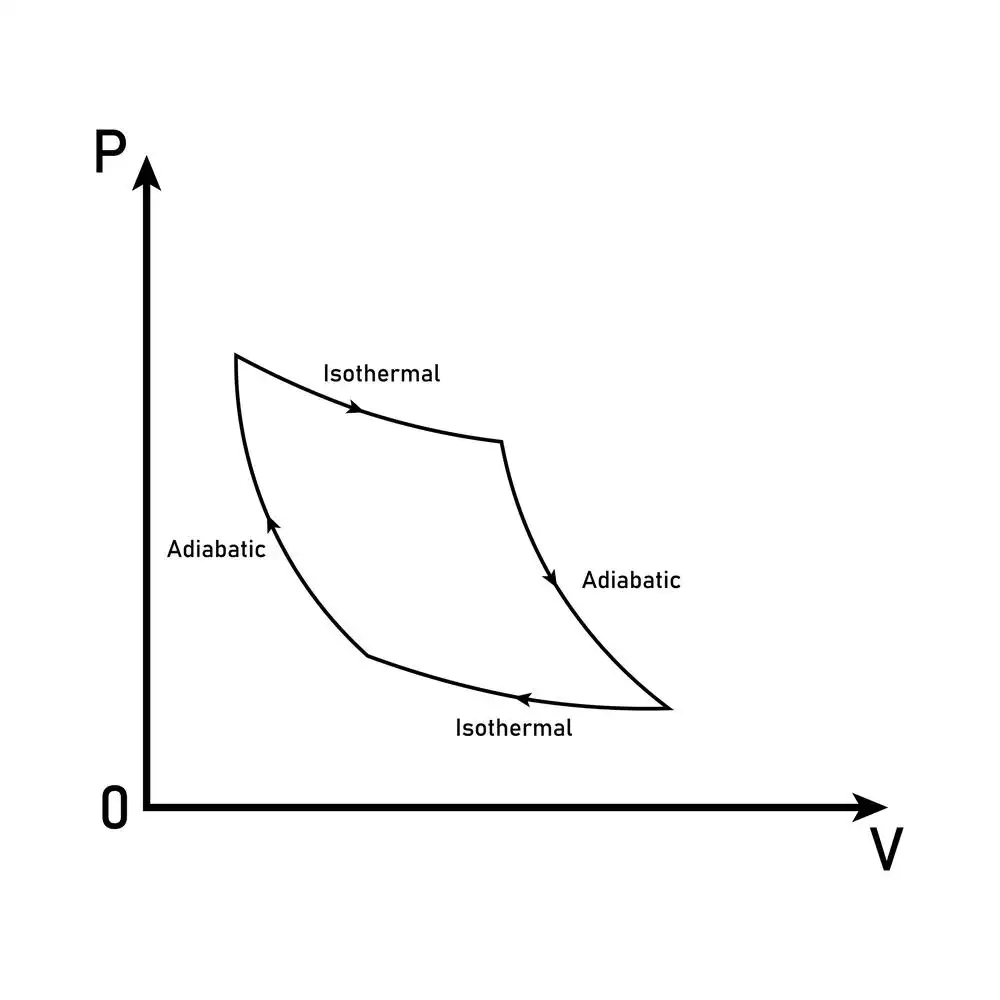 Isotherms and adiabats
Isotherms and adiabats
Thermodynamic system
A thermodynamic system is defined as a collection of matter confined within specific boundaries. Within the system, transformations can take place internally. At the same time, interaction with the outside world can occur through the boundaries, which can have a definite permeability.
Based on the type of the boundary of the thermodynamic system, it can be classified into three categories: isolated system, closed system, and open system.
- In an isolated system, no energy or matter is exchanged across its boundaries, and no work is performed. An example is items packed in a vacuum bag.
- A closed system allows for energy transformation, but matter cannot cross its boundaries, as seen in a cylinder closed by a valve.
- And finally, an open system allows for the free exchange of energy and matter across its boundaries, such as a pool filled with water.
Thermodynamic processes
Thermodynamic processes involve an energy transfer between two systems or between systems and their surroundings, resulting in changes in volume, pressure, or temperature. Depending on the type of process, the process can change the energy of the system and perform some work on or by the system.
To classify thermodynamic processes, we look at how they are performed and what conditions they are performed in.
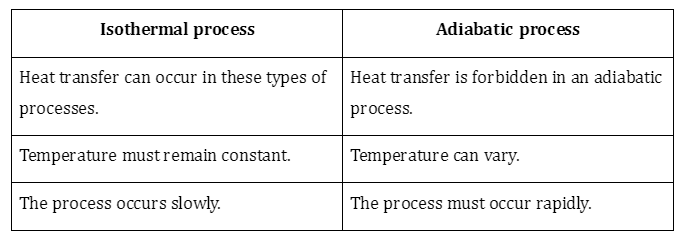
- An isothermal process is one where the temperature of the system remains constant while energy is exchanged in or out.
- An isobaric process keeps the pressure constant.
- An isochoric process occurs when there is no change in the volume of the system.
- Finally, in an adiabatic process, no heat is exchanged by the systems. We’ll discuss adiabatic processes in detail here.
What is an adiabatic process?
An adiabatic process refers to a thermodynamic process in which, no heat energy is exchanged between the system and the surroundings. To be considered adiabatic, the system must satisfy two conditions.
- It must be completely isolated.
- The process must occur over a short enough time frame, which makes it impossible for heat transfer to take place.
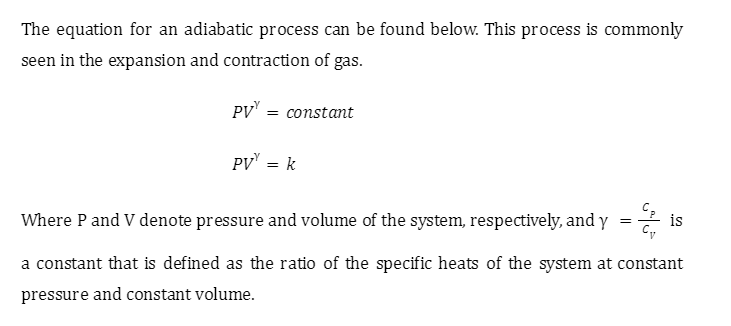
Work done in adiabatic process
We start with a cylinder with walls made up of an insulating material. We assume that it has a frictionless piston attached to it also made up of an insulating material, effectively preventing heat from escaping.


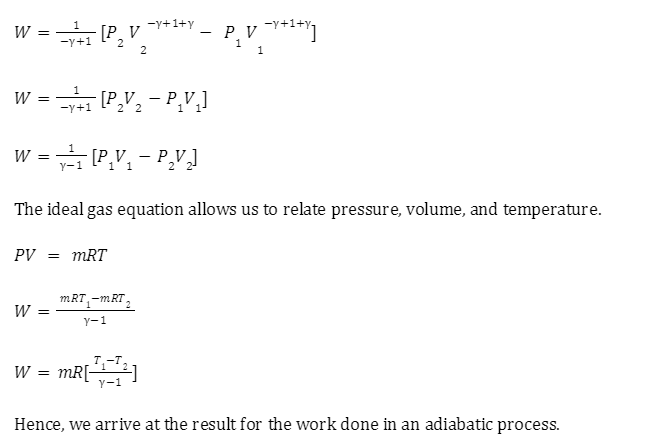
Reversible adiabatic process
An adiabatic process is considered reversible if the system can go back to its original state with no alterations. However, this can’t be achieved practically since a reversible adiabatic process does not really exist in nature. But theoretically, such a process is known as an isentropic process and one example of such a process is the adiabatic expansion of a real gas.
Irreversible adiabatic process
If the system cannot return to its original state after the process has occurred, the process is termed irreversible and is accompanied by a change in the entropy of the system. One example would be heat transfer.
Application of adiabatic process
Adiabatic processes occur in the following scenarios:
- The principle is used in refrigerators.
- Some processes in a thermal engine are adiabatic in nature.
- Compressors and turbines also work on adiabatic principles.
- Igloos and thermos flasks are isolated systems and they utilise adiabatic principles.
- Quantum harmonic oscillators work adiabatically.
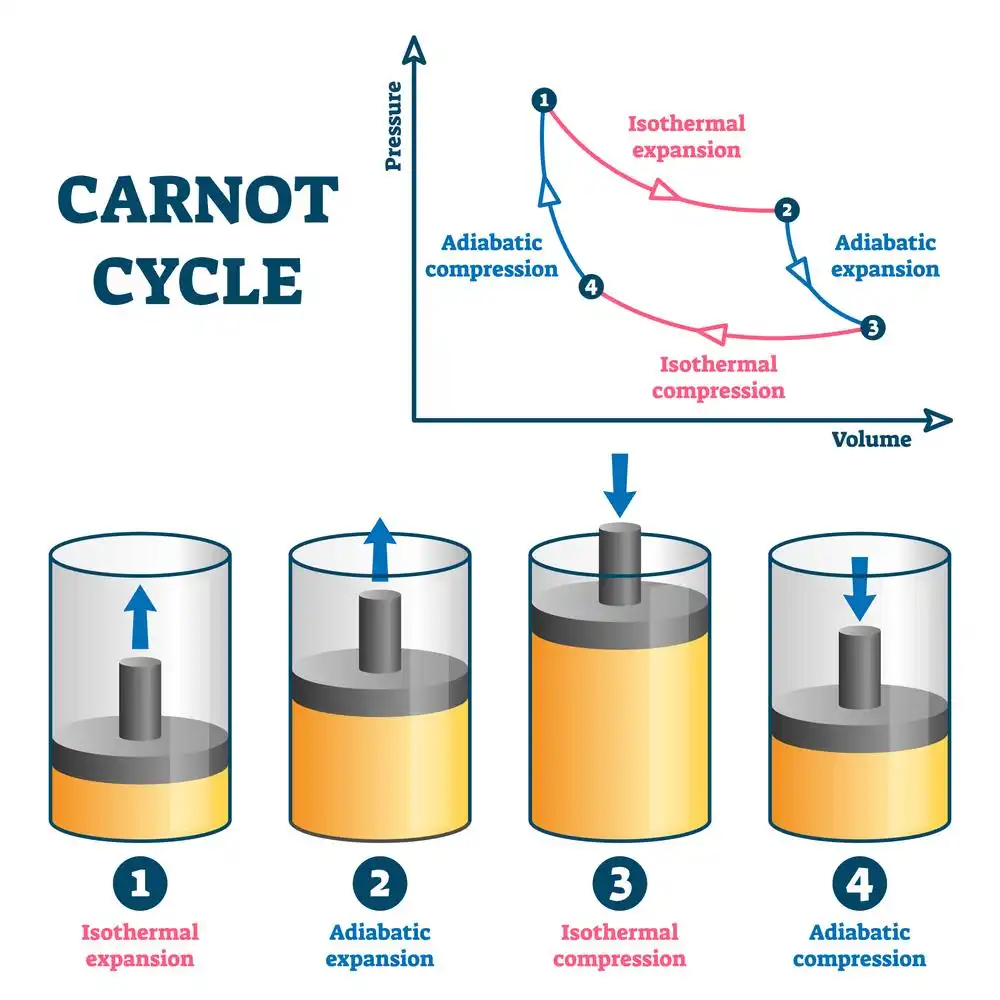
Carnot cycle
Summary
This tutorial covered the topic of thermodynamic systems and processes, including the work done in adiabatic processes. The discussion also included the differences between reversible and irreversible adiabatic processes and the various applications of adiabatic processes.
Frequently Asked Questions
1. Give some examples for all thermodynamic processes.
There are four types of thermodynamic processes: isothermal, adiabatic, isobaric, and isochoric. Some examples of these processes include boiling water, refrigeration, the Carnot engine, heat pumps, the freezing of water into ice, pressure cooking, and vertical atmospheric airflow.
2. Differentiate isothermal and adiabatic processes.
3. What is the specific heat capacity?
The specific heat capacity refers to the amount of energy that must be supplied to one mole of a substance to increase its temperature by one degree.
4. What do adiabatic compression and expansion do?
Adiabatic compression leads to an increase in system temperature and adiabatic expansion causes a decrease in temperature.
5. What is the first law of thermodynamics?
The first law relates the internal energy of the system with the heat exchange the work done. According to the first law, internal energy is the difference of the latter two quantities.
Also Read: Adiabatic Processes Derivation