Introduction
An atom is the smallest component of any given element. Subatomic particles like as the proton, neutron, and electron can be further isolated from atoms, which were once thought to be invincible. Since the quantity of protons and electrons in every atom is the same, every atom is non-conducting.
When an atom loses or gains an electron, the resulting change in charge is noticeable. These charged particles are called ions. Either by gaining electrons (in which case they are called anions) or by losing them (in which case they are called cations), atoms and molecules acquire or lose their charge. The atomic theory’s central idea is that atoms are the smallest building blocks of matter. None of the simplest chemical compounds or elements are capable of decomposing any further.
Atom
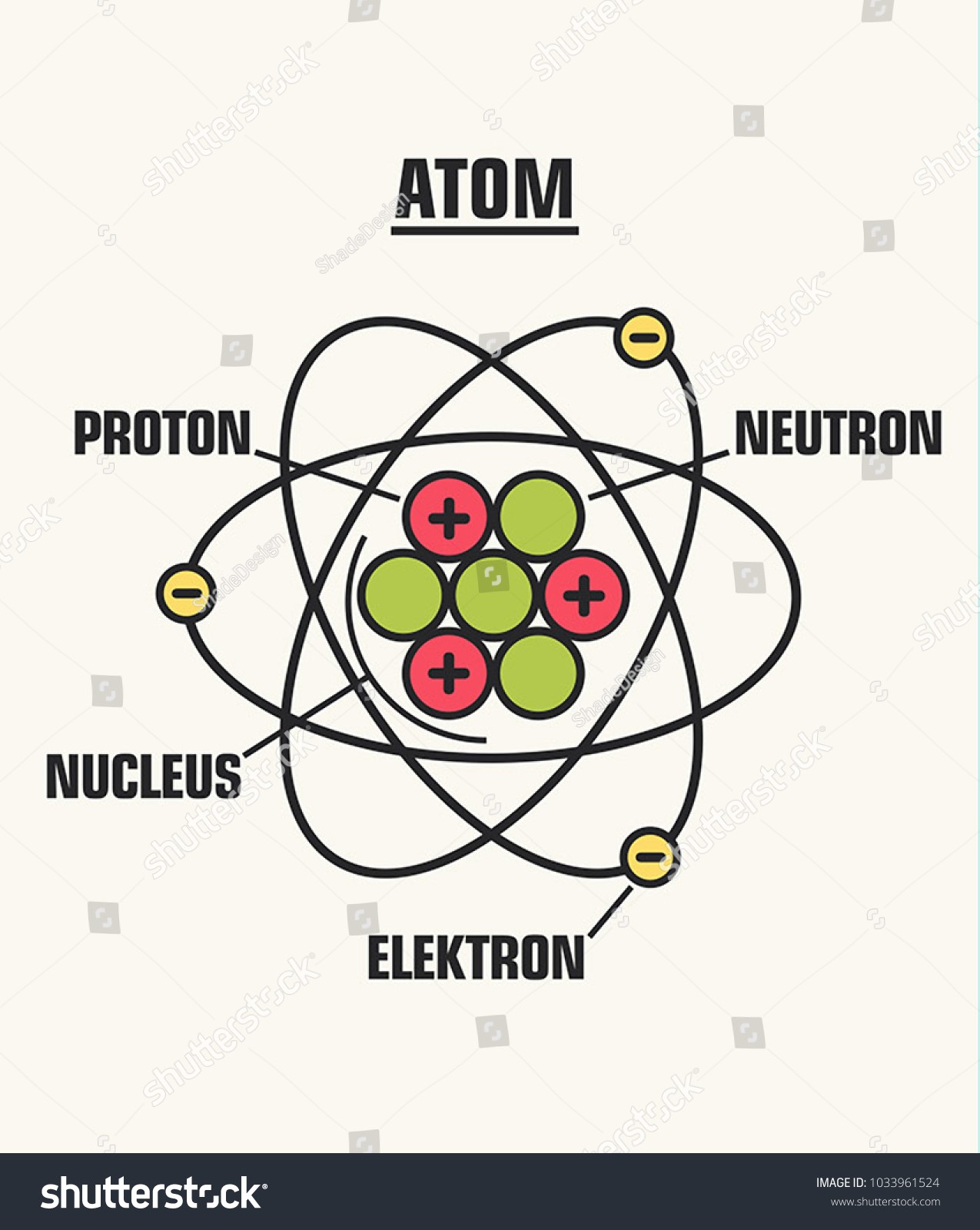
A nucleus, which is positively charged, is packed closely with electrons, which are negatively charged, to form the smallest unit of an element called atom. The structure of an atom, on the one hand, and the additional nucleus region, on the other. The neutron (n°) and the proton (P+) make up the atomic structure. Negatively charged electrons are housed in the supplementary nucleus (e-).
All elements and compounds, including atoms, have mass. The protons in an atom’s nucleus are largely responsible for the extreme density of matter there. The proton is the most massive subatomic particle, followed by the neutron and then the electron.
An electron orbits the nucleus of a hydrogen atom, which contains a single proton. Hydrogen is the most lightweight element.
The nucleus of each atom has a specific amount of protons, and these protons attract a matching number of electrons, rendering the atom electrically neutral. Ions can be created by either adding or removing electrons from atoms. A few examples of these elements are hydrogen, nitrogen, oxygen, and iron.
Features of atom on the bases of modern atomic theory
- The term “modern atomic theory” is used to describe the most up-to-date, canonical explanation of atoms.
- According to the foundations of atomic theory, atoms are the smallest units of chemical matter. They are the most basic building blocks of chemistry; they cannot be broken down any more.
- Each element has its own distinct atomic structure, which differs from that of every other element.
- Although, atoms can break down into much smaller particles. The nucleus of every element contains the same amount of protons, which are positively charged subatomic particles.
- Neutrons are also present in the nucleus, albeit the exact number varies amongst isotopes of the same atomic type.
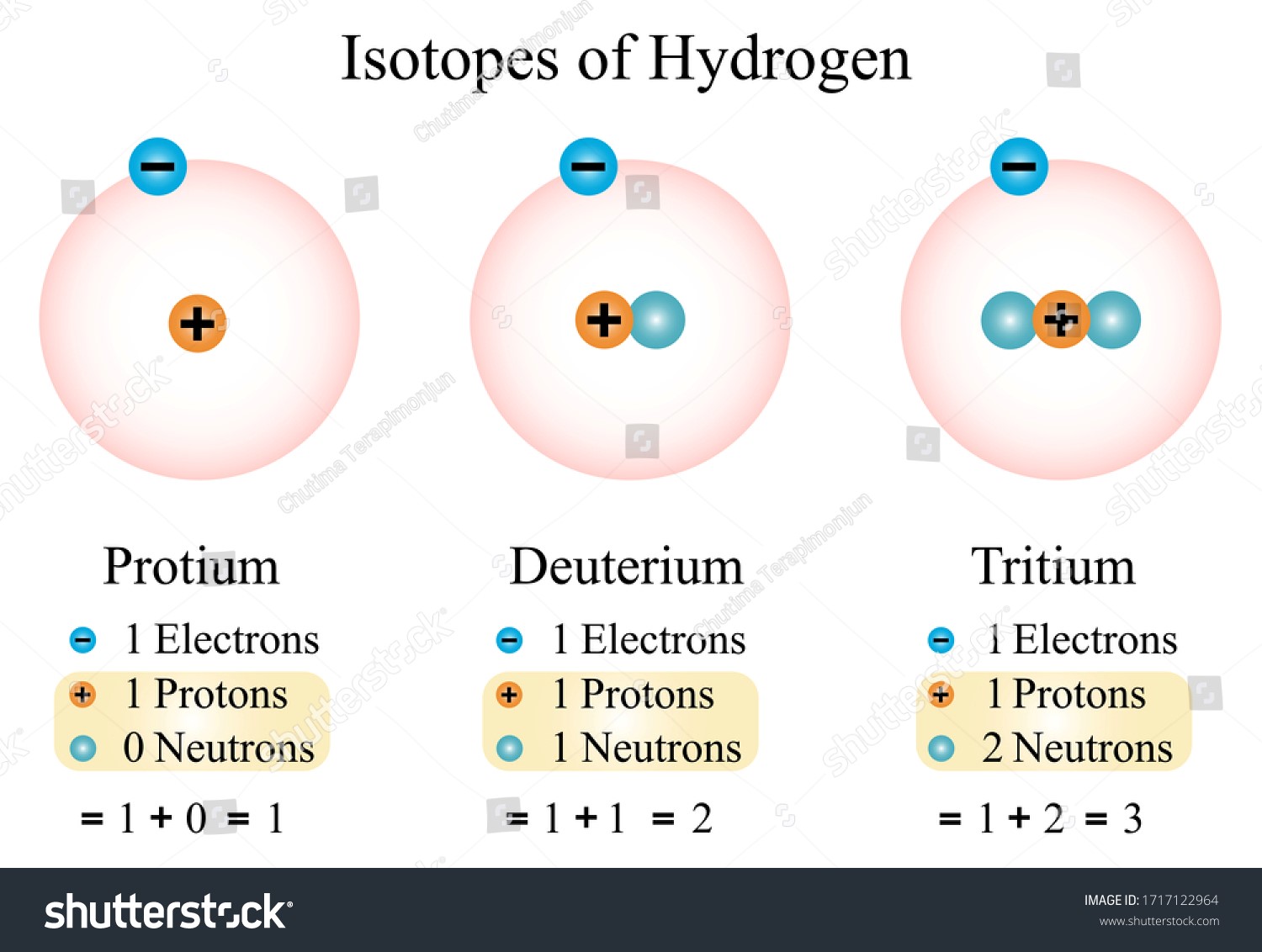
- There are two types of atoms in the universe: isotopes, which have a varied number of neutrons but the same number of protons. For example, whereas all hydrogen atoms share a single proton, hydrogen-2 also has a neutron while hydrogen-1 does not.
Ions
When the number of protons and electrons in an atom becomes unbalanced, ions form. Common charged particles include ions. An ion could have either a positive or a negative charge. If an atom has an electrical charge, it is said to be an ion. An anion is an atom in which the number of electrons is greater than the number of protons. When there are more protons than electrons in an atom, we call it a cation. It’s doable without any outside help. In the process of gaining or losing electrons, an atom becomes an ion. Ions can be divided into two categories: anions (-) and cations (+).
When an atom receives an electron, its electron count rises; as a result, it acquires a negative charge. When an atom loses an electron, it receives more protons than it loses, giving the atom a positive charge.
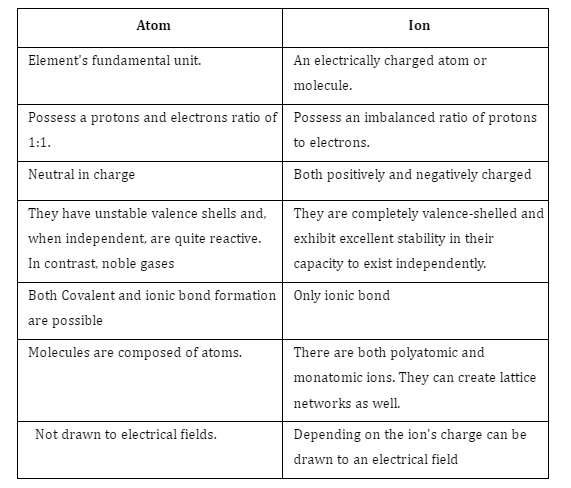
Difference between Atom and Ion
Summary
The contemporary atomic theory suggests that there are two components to an atom. The nucleus and the atomic orbitals. Electrostatic repulsion does not exist between protons and neutrons, hence the nucleus is composed of both types of particles. All stuff is composed of smaller and smaller particles called atoms. Subatomic particles can change into ions by gaining or losing an electron. Ions are sometimes mistaken for atoms, but not always; some compounds can undergo an electron-loss or -gain transformation to become ions. Ions have a net electrical charge, while atoms do not; this is the main contrast between the two.
Frequently Asked Questions
1. What is the function of the nucleus in an atom?
Ans. The nucleus of an atom contains the vast majority of the atom’s mass in the form of protons and neutrons. These two hold down the nucleus. The electrons orbit the nucleus.
2. Does the property on an ion differ from its parent atom?
Ans. Ions have different electronic configuration than their parent atoms. It results in different chemical properties because of the presence of charge. It also differs in terms of size.
3. Who discovered atom?
Ans. Democritus invented the atom in 450 B.C. He separated a matter into smaller and smaller fragments until it could no longer be divided. He called them atomos, afterwards renamed atoms. John Dalton revives Democritus’ hypothesis and performs several experiments to establish atoms exist.