Introduction
The term “fluid” is used to describe a material that may take on several forms. Things that are fluids are ones that can be moved around rather easily. This chapter focuses on the physical properties of viscosity and surface tension shown by fluids. The two processes are dependent on molecular interactions. Both surface tension and viscosity are measures of a fluid’s elasticity; the former is responsible for the fluid’s relatively small surface area, while the latter indicates how much is its fluidity.
What is Surface Tension?
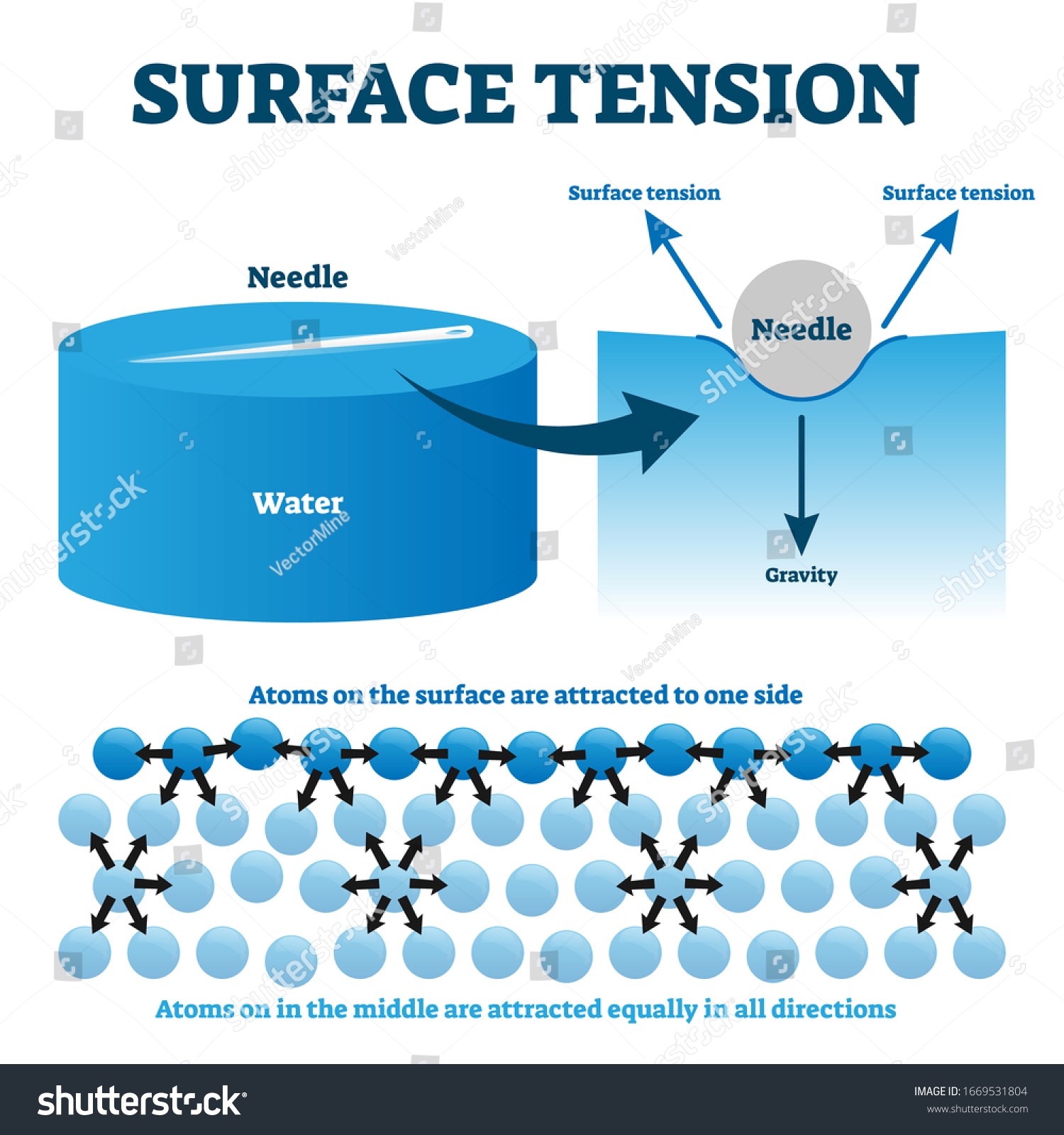
- Surface tension is a fluid’s tendency to take on the smallest possible footprint on a surface.
- Liquids have this quality because the molecules near the surface are in a different state from the molecules deeper in the substance.
- When a molecule sinks below the liquid’s surface, it is surrounded by other molecules and experiences equal attraction in all directions.
- Therefore, the molecule is not being attracted by any net force.
- Surface tension is affected by the attractive forces exerted by the surrounding solid, liquid, and nearby particles, as well as those exerted by the particles themselves.
- As the temperature is increased, the surface tension and the net force of attraction between molecules are both diminished.
- The energy needed to increase the liquid’s surface area by one unit is released by surface tension. The fundamental characteristic of the liquid surface that resists force is also surface tension. In particular, it maintains a barrier between the liquid and foreign objects, and it also acts as the force holding the molecules of liquid together.
Applications
- Surface tension is a major factor in many manufacturing processes.
- All businesses with a research and development department use surface tension phenomena to better their products.
- Among the various methods used to raise the standard of production is the development of new detergent formulas.
- Detergent formulations that incorporate more biological surfactants allow for more effective cleaning at lower temperatures.
- Characterizing food, medicine, and packaging all rely heavily on surface tension data.
- Raindrops are spherical because of the cohesive connections between the precipitation molecules and the surface tension of the water molecules.
- Detergents are helpful due to their property of su
- Adding soap or detergent to water lowers the surface tension of the liquid, allowing the water molecules to permeate the fibres and wash away the oil and liquid wax.
- Oil’s lower surface tension than water makes it easier for it to spread across the water’s surface.
- Mosquito eggs can float on the water’s surface because of the water’s surface tension.
- Soap is often included in toothpaste formulations because it reduces the product’s surface tension, allowing for easier distribution.
What is Viscosity?
- Viscosity is a measure of resistance to a fluid’s capacity to flow.
- The resistance to the movement of a fluid, or its viscosity, is the result of friction between the molecules in that fluid.
- Fast-moving fluids have less internal resistance than slow-moving fluids. This is because of the intense intermolecular forces at play.
- Those liquids that move very slowly have a high internal resistance. The cause of this is the weak intermolecular forces present between them.
- An rise in temperature reduces the viscosity of liquids but raises that of gases. Therefore, heat makes liquids more pliable, whereas gases become more resistant to change in velocity.
- When a liquid’s viscosity increases, its flow rate decreases.
Applications
- The highly viscous fluid is used as brake oil in hydraulic brakes and to dampen the movement of a variety of instruments.
- The way blood flows through arteries and veins is regulated by the viscosity of fluids.
- When it comes to lubricating the moving elements of heavy equipment, oil with a high viscosity coefficient is your best bet. Insight into the viscosity of a lubricant and how it changes with temperature can help us select the most appropriate option.
- To find out how much an electron weighs, Millikan used the oil-drop experiment. Because of his expertise in viscosity, he was able to calculate the potential energy.
Summary
The viscosity of a fluid is directly proportional to the amount of friction it encounters as it moves through a given space. The resistance to motion in a fluid, known as its viscosity, is caused by friction between the molecules of that fluid. Internal resistance is lower for fluids that are moving quickly. This is because of the intense intermolecular forces at play. The tendency of a fluid to leave as small a footprint as possible on a surface is an example of surface tension. Liquids have this property because molecules at the surface are in a different state from those deeper in the material.
Frequently Asked Questions
1. What is the difference between dynamic and kinematic viscosity?
Ans: The friction between two layers of a fluid during motion is known as its dynamic viscosity. As most cases, it will be expressed in centipoise. The dynamic viscosity of a fluid is converted into a kinematic viscosity by dividing it by the fluid’s density.
2. How is viscosity measured?
Ans: The viscosity is determined using the viscosity coefficient. This value is independent of the specific liquid being analyzed and remains constant over time. Formally, the coefficient of viscosity is estimated using the Poiseuille’s method, in which the liquid flows through a tube at various pressures.
3. What benefits does viscosity in vehicle industry?
Ans: Increases in viscosity, brought on by higher temperatures, tend to lessen wear and oil consumption. A decrease in viscosity caused by cooler temperatures improves ignition and reduces fuel use.