Introduction
Chemical reactions operate on the type of reaction and change principle, which states that when a reactive substance interacts with other chemicals, different reactions will occur. Chemical reactions occur in stable or least reactive compounds as well, albeit under more extreme conditions. When reactants combine to produce a product, heat is released or absorbed, bubbles, gas, and fumes are formed, and the colours of the reactants change, resulting in a chemical change. During a physical change, there is an interconversion of conditions. There are various types of chemical reactions, such as combination, decomposition, and displacement reactions, and certain changes occur during these reactions.
What is a Combustion Reaction

A combustion reaction is an exothermic chemical reaction that occurs between a fuel (or reductant) and an oxidant and results in the formation of oxidised products. This process occurs at high temperatures. This is a redox reaction because it involves the simultaneous reduction and oxidation of substances. A struck match, for example, generates friction, which raises the temperature (or energy) of the head (more than activation energy), at which the chemicals react and generate more energy in the form of heat, which tends to escape into the atmosphere. A moist matchstick head or the presence of blowing wind prevents the temperature from rising. As a result, the matchstick’s head does not burn. Another common example of combustion is the combustion of fuel in automobiles, which produces smoke.
Examples of Combustion Reaction
Methane combustion
Methane is a natural gas that burns the cleanest of all fossil fuels. The term “cleanest” refers to the absence of harmful toxins. It completely degrades into water and carbon dioxide.
Butane combustion
Lighters use the combustion process to break down butane. Butane is significantly less expensive than other fossil fuels. This is also a clean fuel, but it emits a lot of carbon dioxide into the atmosphere.
Butanol combustion
Butanol combustion occurs during the transportation process. Butanol has a high energy density and a low vapour pressure. As a result, it qualifies as a biofuel. Internal combustion engines, or IC engines, use this combustion process.
What is a Displacement Reaction
Displacement reactions occur when a portion of one reactant is replaced by another. A replacement reaction is another name for it. As one reactant ion is replaced by another. Single displacement reactions occur when one element removes another from its salt or complex. Single replacement reactions are another name for them. General representations can also be written –
\[A + B – C{\rm{ }} \to {\rm{ }}A – C + B\]
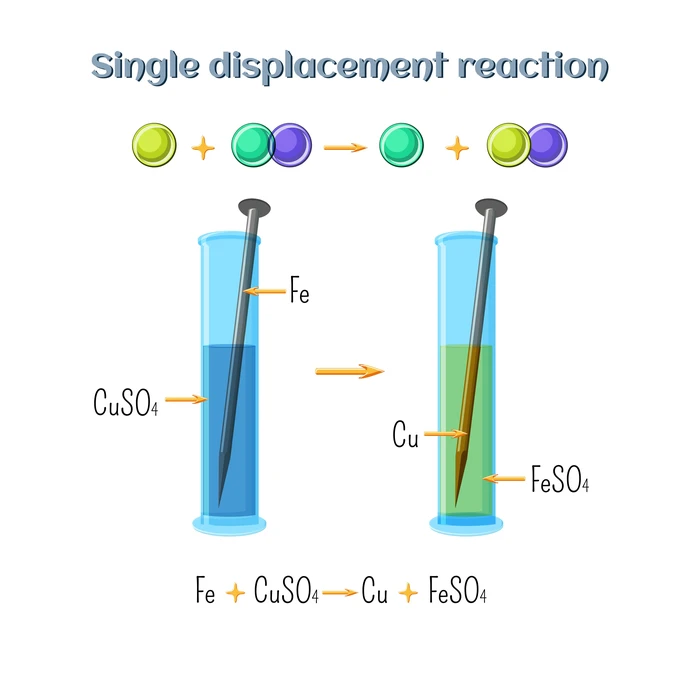
Examples of Displacement Reaction
- The reaction between Calcium Iodide and Chlorine.
\[Ca{I_2} + C{l_2} \to {\rm{ }}CaC{l_2} + {I_2}\]
- The reaction of Zinc with Hydrochloric Acid.
\[HCl + Zn{\rm{ }} \to {\rm{ }}ZnC{l_2} + {\rm{ }}{H_2}\]
Decomposition Reactions
A decomposition reaction is a chemical reaction that occurs when one reactant breaks down into two or more products.
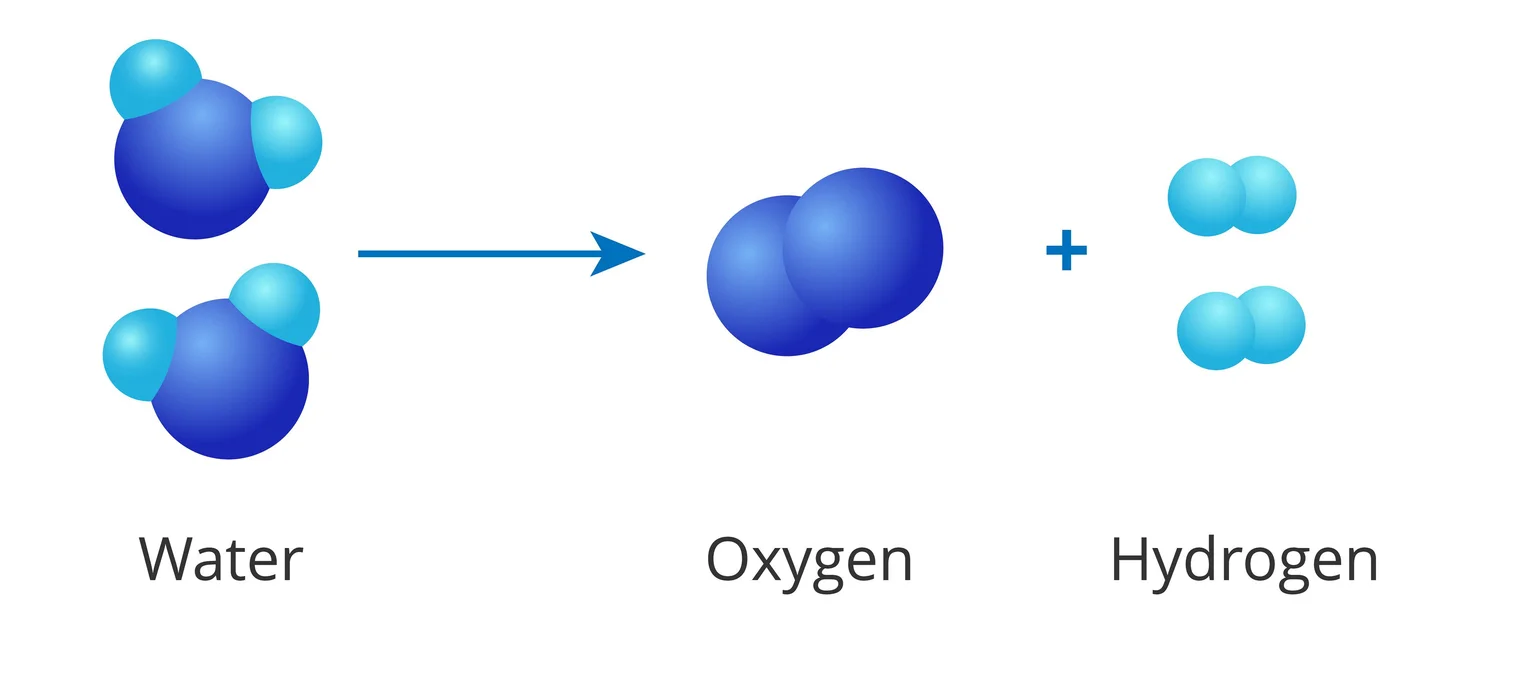
The 2 main categories of these reactions are as follows
- The reaction of Thermal Decomposition:
It is a decomposition reaction that is triggered by heat energy.
E.g. \(CaC{O_3} \to {\rm{ }}C{O_2} + CaO\)
When heated, calcium carbonate breaks down into calcium oxide and carbon dioxide. Quick lime, a crucial component in several industries, is made using this process.
- The reaction of Electrolytic Decomposition:
Electrical energy is used to give the activation energy for a breakdown in an electrolytic decomposition process. An electrolytic breakdown reaction, such as water electrolysis, is exemplified by the chemical equation:
E.g. \({H_2}{O_2} \to {\rm{ }}{H_2} + {O_2}\)
Summary
It can be concluded that a displacement reaction occurs when one reactant is partially displaced by another. Displacement reactions are also known as replacement or metathesis reactions. The two types of displacement reactions are double and single displacement reactions. In a process known as double displacement, cations and anions in the reactants exchange partners to generate products: When a single reactant partially replaces another, a single displacement reaction occurs.
Frequently Asked Questions
1. What are combustible substances?
Ans. The substances that are easily flammable and undergo combustion are called combustible substances. For Example – LPG, CNG, wood, paper, clothes, etc.
2. What causes an exothermic displacement reaction?
Ans. When one element in a molecule is replaced by another, a single-displacement reaction takes place. A chemical reaction either releases or absorbs energy. If energy is released during the process, it is exothermic. The bond formation is an exothermic process.
3. Mention two uses of decomposition reaction.
Ans. Two uses of decomposition reaction are-
- This is used in the formation of cement or calcium oxide.
- This is also used for welding purposes.