An Introduction to Chromatography
The Greek words chroma, which means “colour,” and graphein, which means “to write,” are the roots of the word “chromatography.” In this procedure, the mixture to be separated is applied to a stationary phase (solid or liquid), and a pure solvent—such as water or any gas—is then allowed to slowly travel across the stationary phase, transporting the components separately based on their solubility in the pure solvent.
Principle of Chromatography
The chromatography principle is that “a mixture is applied to the surface or into a solid, and the fluid stationary phase (stable phase) separates from each other while moving with the help of the mobile phase”.
How to Separate Components from Black Ink
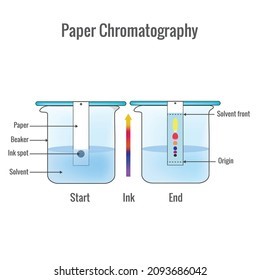
- A thin strip of filter paper is cut out and a line is drawn 3 cm above the lower end of the filter paper. This is referred to as the reference line.
- In the centre of the drawn line, a small dot of black ink is placed.
- When the dot of black ink is dry, it is lowered into the water-filled chromatography jar.
- The filter paper should be immersed so that the black ink dot is above the water level in the jar.
- The setup should be left alone for a while.
- The component of black ink that is more soluble in the water rises faster and higher up on the filter paper as the water begins to rise from the lower end of the filter paper. The level to which water rises is referred to as the waterfront.
- Some coloured spots are observed corresponding to the separated components of the black ink, depending on the number of components present in the black ink.
Summary
The sample mixture is dissolved as the solvent rises through the paper, and it then travels up the paper. Because of differences in solubility and attraction to paper, smaller particles travel further than larger particles.
Frequently Asked Questions
1. What are the advantages of Chromatography?
Ans. The advantages of chromatography:
- A very small quantity of the substance can be separated.
- Components with very similar physical and chemical properties can be separated.
- It defines the different constituents of a mixture.
- It also helps in the quantitative estimation of components of a mixture.
2. What do you mean by Heterogeneous solution?
Ans. A heterogeneous mixture can be defined as a solution with a non-uniform composition, such as dye, milk, and sand water solution.
3. What are the different types of dyes?
Ans. The natural dyes are henna, walnut shells, turmeric, and catechu. Some synthetic dyes are methyl orange, methyl red, congo red, malachite green, rosaniline, pararosaniline, crystal violet, phenolphthalein, indigo, fluorescein, and anthraquinone dye.