Introduction
When a phone is hooked to its charger, how does the battery work? How does the cell in a TV remote control work? All of these questions have been thoroughly explored in the scientific discipline of electrochemistry. Electrochemistry is the study of both the creation of electricity through chemical processes and the use of electricity to conduct non-spontaneous chemical reactions. The task is accomplished by using cells. Cells are the building blocks that trigger chemical processes that produce or generate electricity.
Types of cells
There are two types of cells:
- Electrochemical cell: An electrochemical cell is a device that may produce electricity through chemical processes that occur naturally. The chemical reactions occurring here are called redox reactions. During redox reactions, electrons are exchanged between different chemical species. Galvanic or voltaic cells are other names for these devices. One type of electrochemical cell is the Daniell cell.
- Electrolytic cell: Electrolytic cells are a subset of electrochemical cells that are capable of using electrical energy to catalyse chemical processes. That is to say; electricity needs to come from somewhere else. Then, an artificial reaction can be initiated. Electrolytic cells have traditionally been used for the electrolysis of substances.
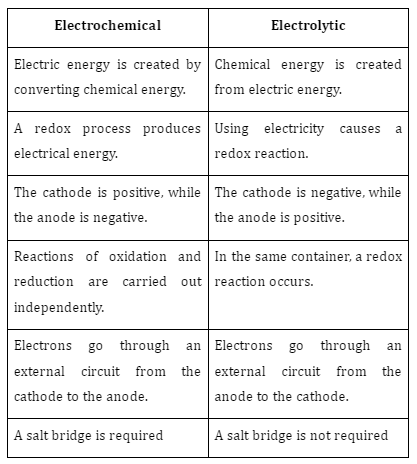
Difference between electrochemical cells and electrolytic cells
Cell structure
There are two electrodes in a cell: the cathode (positive) and the anode (negative) (-ve terminal). Submerge both electrodes in the corresponding metal salt solutions. The anode of an electrochemical cell is also known as the oxidation half-cell because of the chemical process that takes place there. The cathode is the decreased half-cell, another name for it. Connecting the two electrodes is a salt bridge, a U-shaped tube filled with gel and an electrolyte.
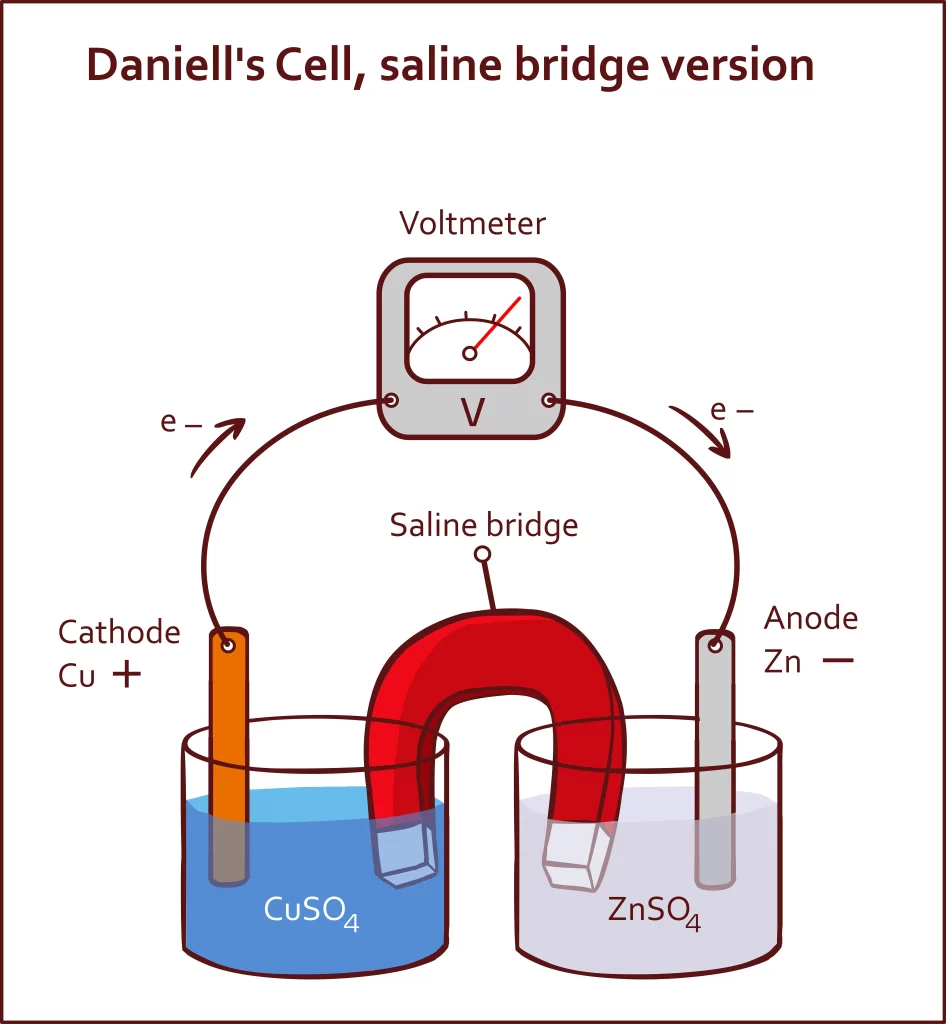
Daniel Cell Diagram
What is a Daniell cell?
An electrochemical cell known as a Daniell cell is used to transform chemical energy into electrical power. The cell undergoes a number of chemical processes in order to produce electricity. Electrodes made of zinc (Zn) and copper (Cu) are used as the anode and cathode in a Daniell cell. The salt solutions have been poured over the metals.
In a Daniell Cell, the anode is composed of zinc (Zn), while the cathode is composed of copper (Cu). There is still a chemical solution containing ions bathing both electrodes. The proper chemicals are copper sulphate and zinc sulphate. It improves the voltaic cell, using its copper and zinc electrodes to produce a 1.1 V potential difference. The cell supplies energy to the circuit after electrons are produced at the anode and transferred to the cathode.
Daniell cell chemical reaction
The following chemical reactions take place in Daniell cell:
\(Zn\left( s \right) + C{u^{2 + }}\left( {aq} \right) \to Z{n^{2 + }}\left( {aq} \right) + Cu\left( s \right)\)
Reaction at the anode:
\(Zn\left( s \right) \to Z{n^{2 + {\rm{ }}}}\left( {aq} \right) + 2{e^ – }\;\;\;\)
Reaction at the cathode:
\(C{u^{2 + }} + 2{e^ – } \to Cu\left( s \right)\;\;\;\)
Daniell cell working
In the Daniell Cell, \(CuS{O_4}\) and \({H_2}S{O_4}\)are stored in a copper container until needed. The components of its operating system are:
- A zinc rod that has crystallised in the zinc sulphate solution may be seen (\(Z{n_2}S{O_4}\)).
- A see-through layer underneath the copper container keeps the \(CuS{{O}_{4}}\) crystals and solution in touch. As a consequence, solution saturation is maintained.
- An electric current is produced by the external circuit.
- Mass is gained by the copper rod and lost by the zinc rod.
- Zinc sulphate concentration rises as Copper sulphate decreases.
- Both methods maintain their electrical neutrality.
Daniell cell representation
The Daneil cell is represented as:
\(Zn{\rm{ }}\left| {{\rm{ }}Z{n^{2 + }}\left( {aq} \right){\rm{ }}} \right|{\rm{ }}\left| {{\rm{ }}C{u^{2 + }}\left( {aq} \right){\rm{ }}} \right|{\rm{ }}Cu\)
The symbol “| |” represent the salt bridge, the right side represents the reduction half-cell, and the left side refers to the oxidation half-cell.
Application of Daniell cell
Some of the applications of daniel’s cell are:
- Used for making batteries, which are essentially just groups of cells in series.
- Creating electricity while minimizing electrical consumption.
- Telepathy through inductive coupling
Summary
Certain electrochemical cells function in an electrolytic fashion. Hence, the electrolytic cell has everything that would normally be present in an electrochemical cell. Both electrochemical and electrolytic cells rely on the transport of electrons throughout the system to carry out their functions. Whereas electrolytic cells undergo non-spontaneous chemical reactions, electrochemical cells undergo chemical reactions of their own accord. To put it another way, an electrochemical cell is not the same as an electrolytic cell.
Frequently Asked Questions
1. What is a rechargeable cell?
Rechargeable batteries can only be made from secondary cells, which undergo reversible chemical processes. a cell that generates an electrical current, but whose chemical activity may be reversed by delivering a current in the opposite direction through the cell.
2. What maintains the electrical neutrality in a cell?
The Salt Bridge is responsible for preserving charge neutrality in the Daniell cell’s two compartments. It is a glass tube which contributes to keeping the balance of the charge.
3. Why are the charges of electrodes different in Daniel cell?
Daniel cell is an electrochemical cell whose anode has a negative potential with the solution. Which makes it negatively charged. The removal of the metals from the anode during oxidation causes a buildup of electrons upon that anode, which gradually turns it negative. Eliminating metal ions from the electrolyte results in the consumption of electrons, which turns the cathode positive.