Introduction
Molecular formula of barium carbonate This substance belongs to the class of inorganic chemicals. This has no aroma or flavour at all. It is a white salt that, like most other alkaline earth metal carbonates, is weak bases in water solution but soluble in most acids (except sulphuric acid). To put it another way, it is one of the most economically or commercially significant barium compounds. While insoluble in tap water, it dissolves somewhat or only partially in carbon dioxide-rich water (\(C{O_2}\)). When combined with other ingredients, it makes a potent rat poison. Other industries that put it to use include those that produce pigments, clay, polymers, plastics, etc. It’s also a key ingredient in making glasses of varying refractive indices.
What is Barium Carbonate?
The chemical formula for barium carbonate, an inorganic chemical substance, is (\(BaC{O_3}\)). In terms of flavour and smell, it’s completely neutral. It looks like regular table salt, just like the other carbonates of alkaline earth metals. It dissolves poorly or not at all in water, although it does so partially in water saturated with carbon dioxide . It also dissolves in most acids, including nitric and hydrochloric acid, but not sulphuric acid. The mineral form of this is known as witherite, and it occurs naturally. Many industrial and commercial applications exist for it. It’s a crucial barium chemical, actually.
Physical Properties of Barium Carbonate
Som physical properties of barium carbonate are-
- It is insoluble in pure water but it is soluble in the water-saturated with Carbon dioxide (\(C{O_2}\)) and it is soluble in most acids except sulphuric acid.
- It has a specific heat of around 0.1448.
- It generally appears as white crystals or white salt powder.
- It has an atom complexity of around 18.78.
Chemical Properties of Barium Carbonate
Some of barium carbonate’s chemical characteristics are as follows:
- Barium carbonate readily reacts with soluble calcium salts to generate Barium sulphate, which precipitates out of the solution, and calcium carbonate. Here is the chemical reaction that occurs while doing so:-
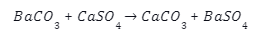
- Barium carbonate can also react with hydrochloric acid to precipitate out as Barium chloride(BaCl), with by-products being water and \(C{O_2}\).
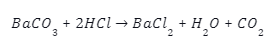
Barium Carbonate Structure
Barium Carbonate
As seen from the above diagram, \(B{a^{2 + }}\) cation is bonded to the \(CO_3^{2 – }\) anion through an ionic bond.
Various Uses of Barium Carbonate
Barium carbonate has a variety of applications, including those listed below.
- As it has been discovered to be an insoluble white salt, it finds extensive application in the ceramics sector, where it is utilised in the production of many different kinds of ceramics.
- Moreover, it is crucial in the production of PTC thermistors, capacitors, and other electronic devices, as well as the increasingly popular electronic ceramics.
- Moreover, barium carbonate is used in the creation of fibre optical glasses and magnetic components.
- It is also a key ingredient in the production of other barium compounds, such as barium oxide and barium peroxide.
- A common method of producing rodenticide also involves barium carbonate (rat poison). It looks like flour or salt, which is probably why rats are drawn to it.
- Barium carbonate has several commercial uses, including in the oil-drilling industry, photography, enamel production, magnetic material production, paint and brick production, and a wide range of chemical processes.
Production Method of Barium Carbonate (\(BaC{O_3}\))
Common production methods are:
Carbonation Method– In this process Barium Sulphide (BaS) is pulverised at high temperature. The reaction takes place as follows:
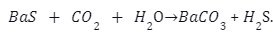
Poison Nepheline Conversion Method– In this method witherite is reacted with an ammonium hydroxide along with ammonium carbonate. The reaction takes place as follows:
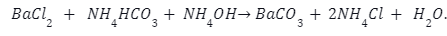
Metathesis Method– In this method barium sulphide and ammonium carbonate react to form barium carbonate through metathesis. The chemical reaction is represented as–
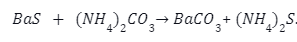
Dry Granulation Method- Here, barium carbonate is sourced from overabundant precipitation, which is then sieved and stored in a raw materials warehouse.
Summary
Inorganic chemical compound barium carbonate is a white crystalline powdered salt. Its molecular formula is \(BaC{O_3}\). It has no discernible taste or smell and no visible hue. In addition to being soluble in carbon dioxide-saturated water and the majority of acids, besides sulphuric acid, it may also be dissolved in pure water. It is important in various commercial industries such as polymer, dyes, paints, etc. Moreover, it is crucial in the production of PTC thermistors, capacitors, and other electronic devices, as well as the increasingly popular electronic ceramics.
Frequently Asked Questions
1. What makes barium carbonate so dependable?
Among common carbonates, barium carbonate has the highest heat stability. A metal’s electropositive nature tends to grow as it moves towards the bottom of the group of alkaline earth metals. As a result, they become more stable at high temperatures. The stronger the influence of the positive ion on the carbonate ion, the smaller the ion must be.
2. Does barium carbonate have any negative effects on the ecosystem?
As barium is insoluble in water and readily combines with other elements found in nature, like carbonate and sulphate, it presents minimal danger to humans and animals. Persistent barium compounds are often found in surface soil as well as the sediment of freshwater soils.
3. Would barium rust if left exposed to the air?
Barium is an odourless, silvery-white metal at room temperature that becomes a silvery-yellow tint when exposed to air. While it cannot be burned, it may break down when heated, releasing harmful chemicals.