Introduction
A chemical is produced when primary alcohol is reacting with a carboxylic acid in the presence of sulphuric acid. The odour of this chemical is fruity and pleasant. The resulting chemical is known as ester. The chemical reaction that results in the creation of the ester is called an esterification reaction. There are three distinct pathways that lead to esterification, by reacting acid anhydride with alcohol, by Chloroform and Alcohol or by acetic acid and ethanol.
What is Meant by the Fischer Esterification Mechanism?
Producing an ester from a carboxylic acid and an alcohol in the presence of a strong acid catalyst is known as the Fischer Esterification Mechanism. Fischer esterification, named for its co-inventors Arthur Speir and Emil Fischer, is also commonly referred to as Fischer-Speier esterification. Alcohol and heat are required for the reaction to take occur. In 1895, they first described the Fischer Esterification process. In most cases, Fischer esterification can be reversed. Common catalysts for the Fischer Esterification Mechanism include sulphuric acid, p-toluene sulfonic acid (PTSA), and Lewis acids like \({C_3}{F_9}{O_9}{S_3}Sc\) (Scandium triflate). The most stable ester appears to become the predominate product due to its latency, suggesting that this is essentially a thermodynamically regulated mechanism. This may be an essential feature when there are multiple reaction hotspots and unwanted byproduct esters to avoid. On the other hand, kinetic controls are commonly employed in reactions involving acid anhydrides and acid chlorides, which proceed quickly.
The Mechanism of Fischer Esterification
In the existence of abundant alcohol as well as a strong acid catalyst, this process transforms carboxylic acids to such an ester as the end product, including water as a residue. There are several stages in the mechanism-
- The acid catalyst acts as a nucleophile of the carbonyl oxygen, allowing it to undergo a nucleophilic assault from ethanol initially.
- The alcohol initiates a nucleophilic attack on the carbonyl. A solitary pair of electrons from the O atom of its alcohol bonds with carbonyl carbon, destroying its π bond with another O atom. The electrons in the bond go upwards to the O, neutralizing their positive electrical charge. It then generates an oxonium ion.
- Following that, a proton exchange from the oxonium ion to the OH group occurs, culminating in quite an active site. It might be divided into 2 stages: firstly, the alcohol deprotonates that oxonium ion, producing the ternary complex, as well as subsequently the OH group adopts the alcohol’s proton.
- Just after 1,2 removal of water, the protonated ester has been produced. A solitary O atom builds a π bond involving the carbon, liberating the water.
- The leftover positively charged O has been deprotonated to produce the necessary ester.
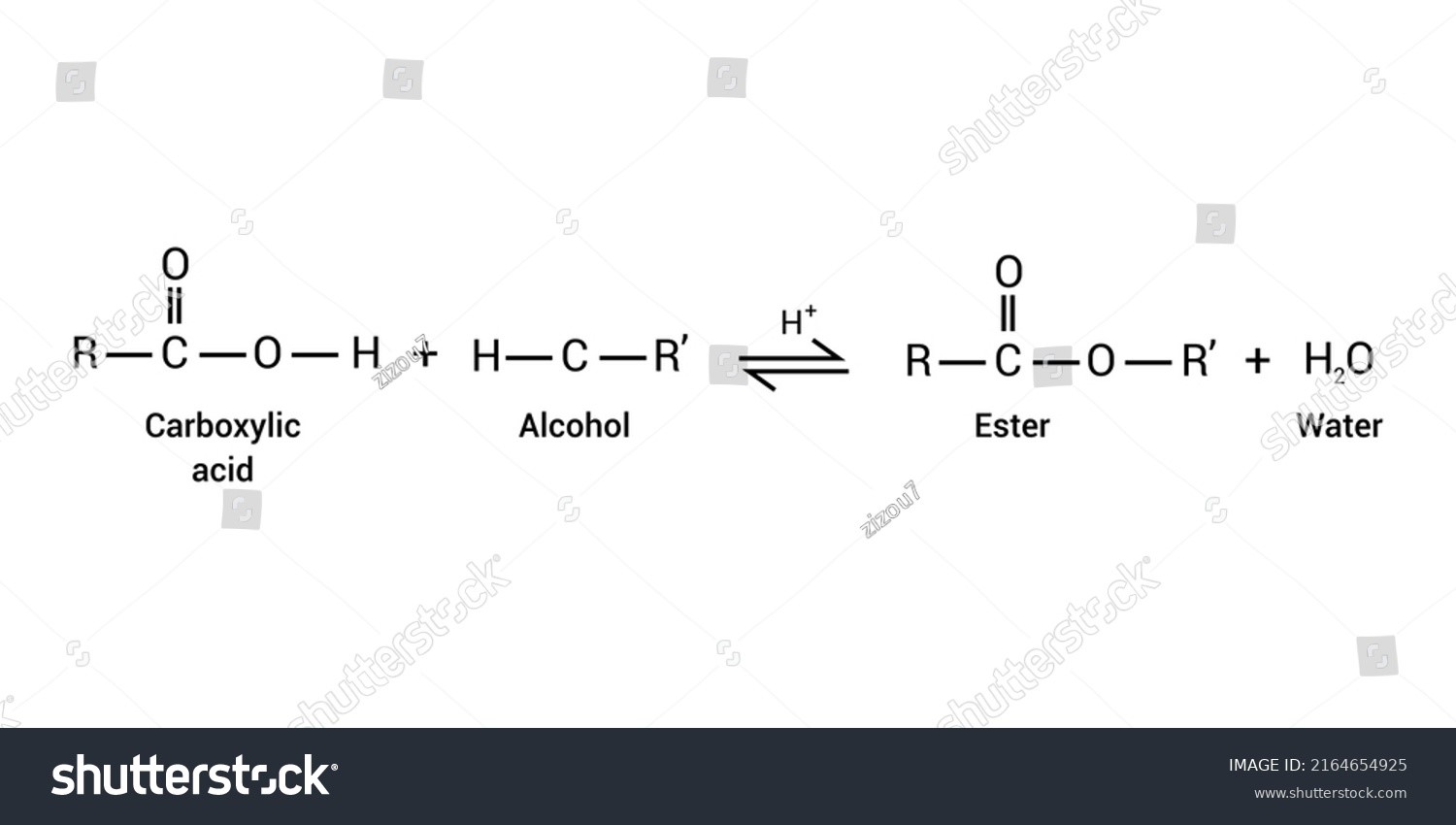
Esterification Mechanism
Advantages of the Fischer Esterification Mechanism
Fischer esterification’s simplicity is one of its primary advantages over more traditional esterification processes. A simple acidic environment can be employed if acid-sensitive functional groups are never a worry; acidic medium could be used; softer acids should be included at the expense of prolonged reaction times.
However, the “direct” nature of the chemicals means that they have had a surprisingly small worldwide impact in terms of garbage and reagent toxicity.
Greenhouse gases or ozone depleters are two of the many environmental hazards that alkyl halides could pose.
The reaction of acid chlorides with atmospheric moisture produces hydrochloric acid gas and they appear to be extremely toxic, but they also react rapidly with water and other nucleophiles; they have been freely quenched from other nucleophiles apart from the preferred alcohol; and even its most popular formulation paths take into account the release of hazardous CO or \(S{O_2}\) gases.
Disadvantages of the Fischer Esterification Mechanism
Fisher esterification techniques have the limitations of being thermodynamically reversible and typically having sluggish reaction rates, which can range from many weeks to years depending on the reaction conditions. When other functional groups appear to be reactive to strong acid, alternatives may be problematic, and new catalytic acids may be selected.
If the generated ester has a lower boiling point than water and the reagents, it can be evaporated in its place. This is a common occurrence, as esters with no protic functional groups have a lower boiling point than their protic parental reactants. The reaction rate is slowed due to the restriction of the overall reaction temperature, but purification and extraction are simplified if the ester product can be purified independently from the reagents. Then, an excess of the starting material is typically required, and the reaction mixture is sealed before being gently heated.
Examples of the Fischer Esterification Mechanism
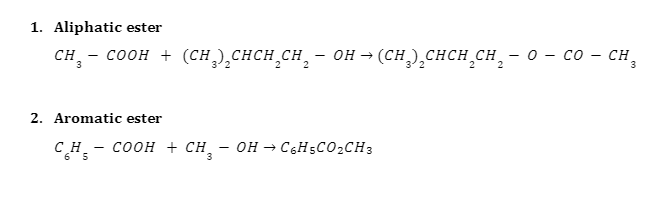
Summary
Indeed, Fischer esterification is a chemical process that changes carboxylic acid into an ester. There is a high concentration of alcohol and an active acid catalyst. Through the course of the reaction, there appears to be an even distribution of products and reactants. The formation of an acyl-enzyme intermediate is the initial step in the Fischer Esterification Mechanism. The ester bond is formed when the alcohol reacts with this intermediate. Ultimately, the ester product is hydrolyzed, releasing the enzyme. An ester has been formed within the presence of water, and this reversible process can only be prevented by continuously eliminating the ester. Fischer esterification’s inability to maintain equilibrium has been one of its drawbacks up to now. However, sulphuric acid is perhaps the most common and efficient acid catalyst throughout the esterification procedure. There should be equal proportions of carboxylic acid and sulfuric acid.
Frequently Asked Questions:
1. What is the role of the deprotonation in Fischer Esterification?
Ans: The deprotonation of the alkoxide ion is necessary for the formation of the ester. It makes the nucleophile strong and favours the reaction kinetically.
2. What drying agent is used in esterification?
Ans:. Concentrated sulphuric acid is the common dehydrating agent used for preparing esters. A carboxylic acid reacts with an alcohol in the presence of conc. sulphuric acid to form an ester. This process is known as esterification.
3. Why is HCl not used in esterification?
Esterifications are not water sensitive, because water is the reaction product. However, for this same reason, the concentration of the water in the reaction mixture affects the equilibrium point. Thus, it is completely unreasonable to use hydrochloric acid as a catalyst for preparative reactions.