Introduction
To separate solids from liquids or gases, chemists employ filtration, which often involves using filter paper or a specialised filtration device. You’ll need a filter to let the liquid through but keep the solids out. The liquid that is recovered after filtration is called filtrate. Due to their inability to pass through the filter’s pores, solid substances are a byproduct of the filtration process that can be discarded. Most filtering processes rely on gravity filtration. The porous media used to retain the solids during filtration is called the filter medium, and the filtered suspension is called the slurry.
Filtration Definition
It’s a physical separation technique that can sort compounds out of mixtures, but not the other way around. It’s one of the simplest ways to separate a solid from a liquid that doesn’t dissolve. A filtering media is needed to separate the particulates from the liquids, which are then collected in the beaker. This filter acts as a liquid-only porous medium, stopping solids in their tracks and letting only liquids pass through. Filter cakes are the dense accumulation of solids on a filter after repeated filtration using the same filtering medium.
Filtration Process
The following method is employed when the particles to be separated are smaller than the pores of the filter media. This means the filter media is effective at keeping the liquids out while discouraging the solids. Additionally, the filter cakes act as a secondary filter by preventing the passage of particular slurries.
- Take water that is to be filtered in a glass beaker.
- Take a funnel.
- Make a little cone out of filter paper & place it on the funnel.
- Finally, strain the mixture through the filter funnel.
- The dirt substances will be visible on the filter paper while the remainder of the liquid has been filtered down.
Understanding the Concept of Filtration
Filtration is the process used to remove solid particles from a liquid or gaseous medium. Solid substances are always present in liquids and gases. By definition, a filter is a media that allows a liquid to pass through but traps any solids within it.
Filtration Diagram
The most frequent method of filtering is to use gravity to settle the substances first. The solution is then placed over filter paper, & the water drops because of gravitational attraction, & the residual stays in the filter paper itself.
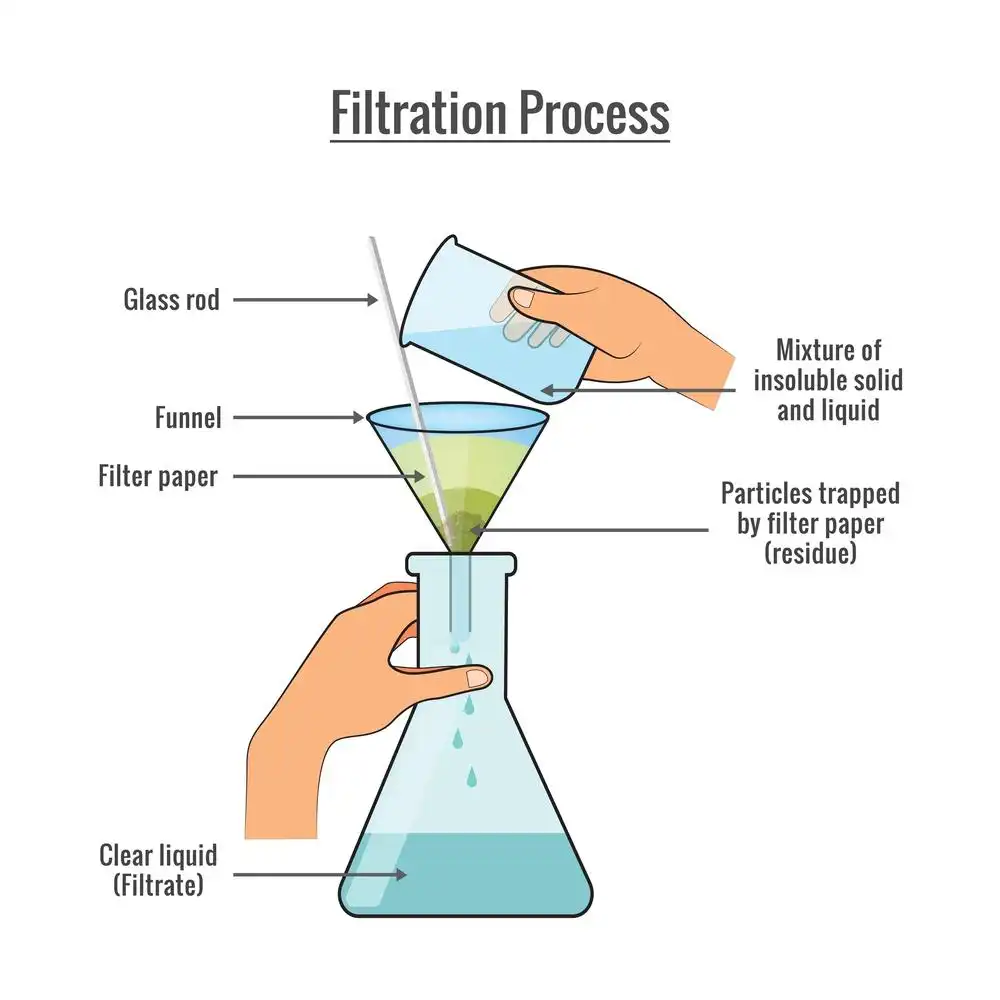
Filtration process
Filters in Use
- Common filtering aids included silica, diatomaceous earth, cellulose, and perlite.
- They have multiple practical applications, either alone or in tandem with conventional paper filters.
- Brewing coffee involves passing hot water through a filter and grounds.
- Coffee fluid is the result of filtration.
- Tea bags (paper filters) are used similarly to accomplish the soaking process.
- Organs that function as natural filters include the kidneys.
- The glomerulus purifies the blood.
- Many vital chemicals are reabsorbed into the circulatory system.
- To get rid of allergens like dust and pollen from the air, HEPA filters are used in air conditioners and several types of vacuum cleaners.
- To remove contaminants from the water, many aquariums use filters made of fibres.
- Belt filters are used to remove precious metals during the mining process.
- Aquifer water is typically safe to drink since it has already been filtered by the earth’s permeable and solid rock.
Applications of Filtration
- Dust is currently collected using vacuums with attached filters.
- In rainwater harvesting, water from iss collected and stored underground. Before being used for drinking and cooking, this water is disinfected in a series of sedimentation tanks and filters.
- Soil, sand, and insects can all be removed from water by filtration.
- By absorbing the essential oils of flowers, fruits, and nuts, many oils become fragrant and nutrient-dense. They are purified and put to use in the pharmaceutical industry.
- In the scientific world, filtration plays a crucial role. Some water-based compounds dissolve in oil, whereas those that don’t leave a residue that can be redissolved and used again by adding oil to the mixture.
Seven Steps of the Water Purification Process
Ion Exchange & Coagulation:
This is the initial stage in the procedure. The water from urban areas is polluted with undesired minerals, & this procedure assists in the removal of the minerals that generate hard water. This method is utilized to separate heavy metals such as iron. The dirt substances are subsequently deposited at the tank’s bottom.
Sedimentation:
The water then travels through the sedimentation procedure after the 1st stage. When the water settles, the floc sinks to the bottom. Sediment filters are used to catch dirt particles. This assists in preventing contamination of the equipment.
Filtration & Granular Activated Carbon:
Water is filtered & then transported through layers of sand, charcoal, & other materials in this procedure. The substances left behind from sedimentation are eliminated. Herbicides, chlorine, & other contaminants are eliminated by a carbon filtering process.
Disinfection:
Water is transported into a closed tank with UV lamps that act as a sterilising agent throughout this operation. If it’s underground water, this procedure is sufficient to purify it since all microbes are destroyed.
Carbon Filters:
Carbon filters are used to adsorb impurities left after disinfection such as coulour impurities.
Reverse Osmosis:
A semipermeable membrane is employed in this case to remove pollutants from the water. All dissolved contaminants left over from the previous processes are eliminated here. In addition, at this step, a sweet flavour is added to the water.
Store Purified Water:
After the above-mentioned procedure, clean water is kept in tanks.
Summary
The process of filtration is used to separate various liquid mixes. It can’t be used to purify chemicals in any way. The addition of a filtration medium component is required. As filtration velocity increases, the filter cake grows thicker. There is no cloudiness whatsoever in the filtrate. Sedimentation, distillation, evaporation, and decantation are only a few of the filtration techniques available. After completing the filtration process, the filters cannot be reused and must be discarded. While the pollutants being filtered out may be harmful, publicly discarding the filters can pose an even greater threat to the environment and its inhabitants.
Frequently Asked Questions
1. What type of carbon is used in carbon filtering?
Activated carbon is used in carbon filtering which has a size of 0.5 to 50 μm. A bed of this charcoal is used to remove impurities through adsorption.
2. How is distillation different from filtration?
Distillation uses an external heat source to change the concerned liquid to gaseous phase and isolate the pure liquid. . Filtration uses specific filters to remove the impurities from the concerned liquid.
3. Which chemical disinfectant is used in water purification?
Chemicals such as bleach, alcohol, phenols, and iodine are used to purify water and kill microorganisms.