Introduction
Alcohol is a common chemical compound. One or more hydroxyl groups (OH) attached to the carbonyl (C) atom of an alkyl group define an organic compound. Ethanol and methanol are the most prevalent types of alcohol, although there are many others. Different kinds of alcohol have different purposes. Although they share the alcohol family with methanol, ethanol is used for very different purposes.
In order to stay safe, it’s crucial to put in lots of research time before picking an alcoholic beverage. Methanol is an alcohol that is used to produce gasoline and other solvents like antifreeze. On the other hand, ethanol is the main component of all alcoholic beverages. Each type of alcohol has advantages and disadvantages in terms of cost, impact on the environment, and potential health risks.
What is Methanol
The formula for this compound is \({CH_3OH}\). . It’s a dangerous variety of alcohol that shouldn’t be drunk. You can find this substance in gasoline, solvents, and even antifreeze; it goes by a few different names. It is also a key ingredient in the manufacture of chemicals like acetic acid. As a byproduct of their metabolism, it can be found in fruits and vegetables.
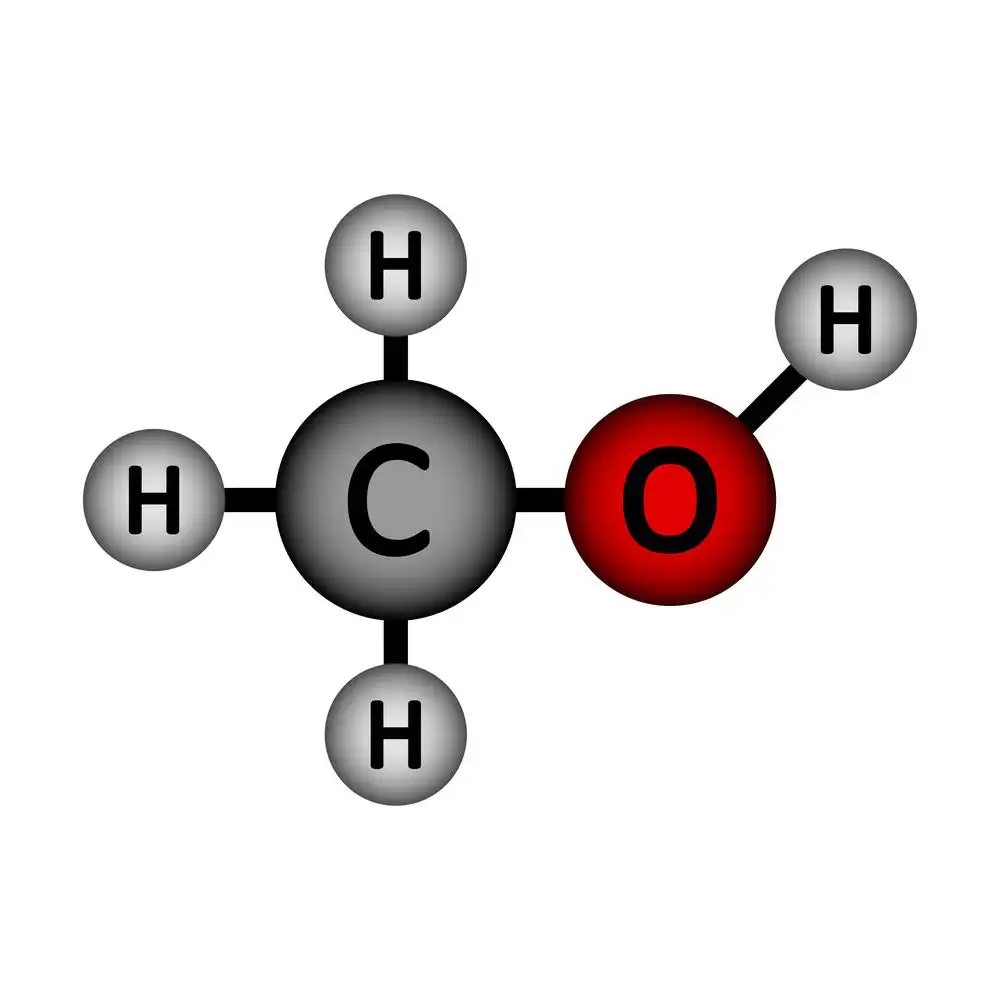
Properties of Methanol
- Methanol has a molecular mass of 32.04 g/mol and a density of 0.792 \(g/c{m^3}\).
- It is a colourless flammable liquid that is volatile
- Methanol freezes at a temperature of 93.9 °C (137 °F) and boils at 64.96 °C (148.93 °F).
- It burns with a dull flame and generates explosive combinations with air.
- It is fully miscibile with water.
- Due to the vapours’ slightly heavier-than-air density, they may return to an ignition source after travelling some distance.
Uses of Methanol
- It is manly use as a raw material for chemical production.
- Methylamine production requires its use.
- It’s also helpful in making acetic acid out of formaldehyde.
- It’s added to liquids to make them freeze at a lower temperature.
- It is used as an engine fuel in high-performance vehicles like sprint cars and even stunt cars when it is in its purest form.
- It is also used as an HPLC solvent and in other laboratory applications.
- In addition to being a fuel and an amphiprotic solvent, it is also a metabolite in humans, mice, Escherichia coli, and Mycoplasma genitalium.
What is Ethanol
It has the chemical formula \({C_2}{H_5}OH\) and is simple alcohol. It is a polar substance. Because of the presence of the OH group, it may also create hydrogen bonds. It’s also known as ethyl alcohol or grain alcohol. It is an important component in beer, wine, and even brandy.
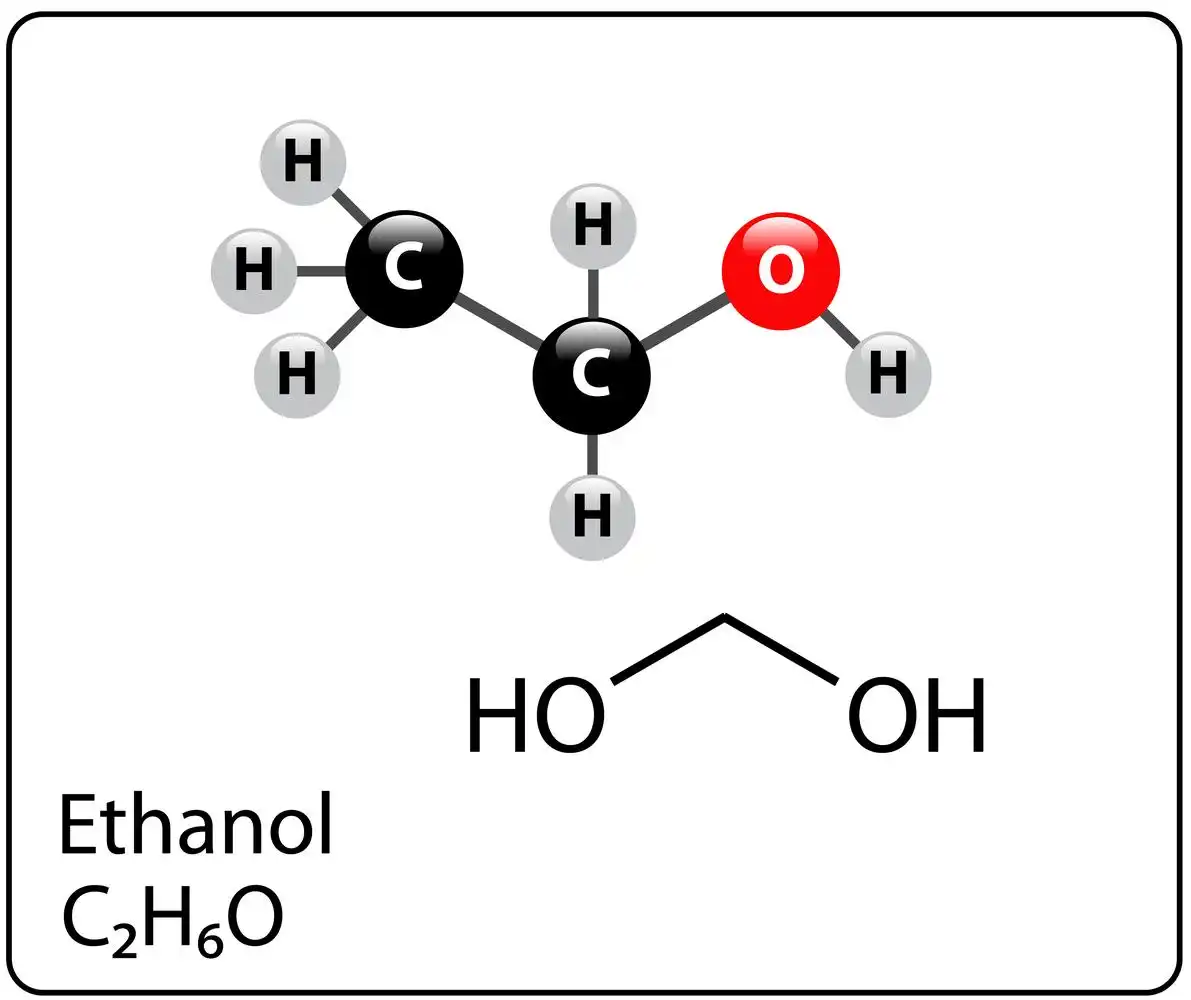
Since ethanol is easily dissolved in water plus other organic substances, it can be found in a variety of different items. This alcohol is a natural outcome of plant fermentation that occurs from ethylene hydration. The sugar fermentation procedure with the zymase enzyme may readily produce ethanol. At low concentrations, it is less hazardous than methanol (\({CH_3OH}\)). However, it is poisonous to the body as well as, in the liver, it turns to acetaldehyde, which is similarly harmful.
Properties of Ethanol
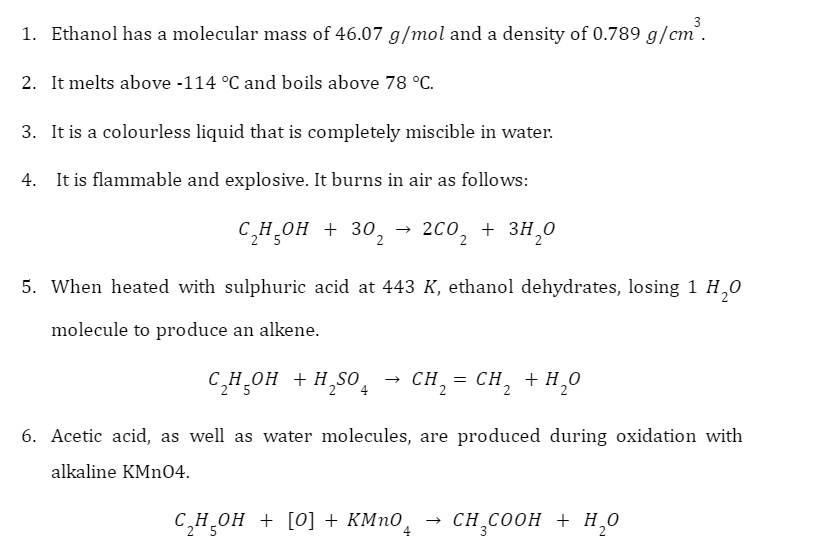
Uses of Ethanol
- Ethanol is frequently employed as a disinfectant and antiseptic.
- Ethanol is commonly used as a treatment for ethylene glycol and methyl alcohol poisonings.
- In many cases, ethanol is used to dissolve drugs that cannot be dissolved in water.
- Some pain relievers and mouthwashes, for instance, use ethanol (in concentrations ranging from 1% to 25%) as a solvent.
- Many alcoholic drinks used orally for enjoyment have ethanol as their major constituent.
- It has the effects of a psychoactive substance, making people feel relaxed and happy.
- However, it operates as a CNS depressive and reduces mental and physical capabilities.
- In the manufacturing sector, ethanol is used to make a variety of products, including ethyl esters, acetic acid, diethyl ether, and ethyl amines.
- Because it can dissolve both polar and nonpolar molecules, this chemical finds widespread application as a solvent.
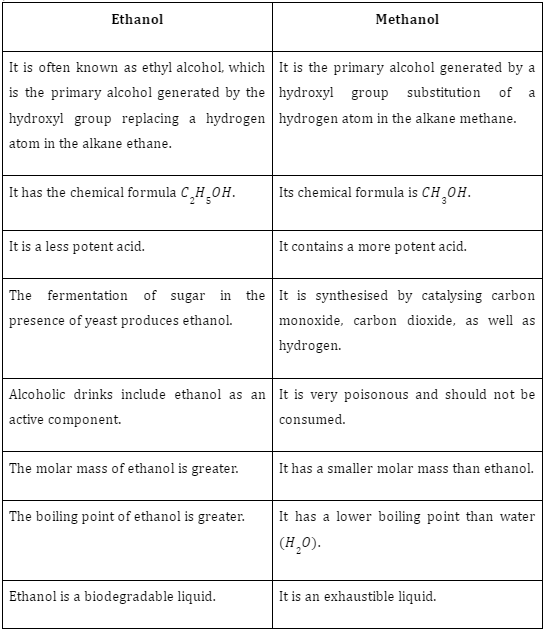
Difference Between Ethanol and Methanol
Summary
Methanol, or \({CH_3OH}\), is an alcohol consisting of only four elements: hydrogen, oxygen, and carbon, making it both water- and biodegradable. It burns cleanly and decomposes easily. It has the chemical formula \({C_2H_5OH}\) and is simple alcohol. Ethanol is an almost colourless liquid with a strong, winey aroma and flavour. Primarily, ethanol is ethane with a hydroxyl group inserted into one of its hydrogens. Both compounds are vital to numerous industries and have widespread application.
Frequently Asked Questions
1. What is ethanol biomass?
Ans. Ethanol biomass is the ethanol produced entirely through various plants. It is produced mainly through the process of fermentation utilizing microorganisms such as bacteria and yeast.
2. Can methanol be created through natural sources?
Ans. Certain bacteria species create methanol spontaneously through anaerobic respiration. Aside from that, we can create it industrially using fossil fuels such as natural gas and coal.
3. Why is alcohol denatured?
Ethanol is often denatured to discourage its recreational use and to make it usable for industrial purposes and fuel manufacturing. Pyridine and methanol are generally used for this purpose.