Introduction
The fundamental building block of life, a cell is capable of carrying out every metabolic task required to maintain life. A cell must be able to permit the passage of various substances into and out of its boundaries in order to be able to perform the metabolism of various substances. A cell can take in and release substances out of the cell, through the process of diffusion and osmosis. This exchange is essential for the cell’s survival. The processes of molecules moving down a concentration gradient are diffusion and osmosis. Although they both aid in the movement of substances through the cell membrane, they differ slightly from one another.
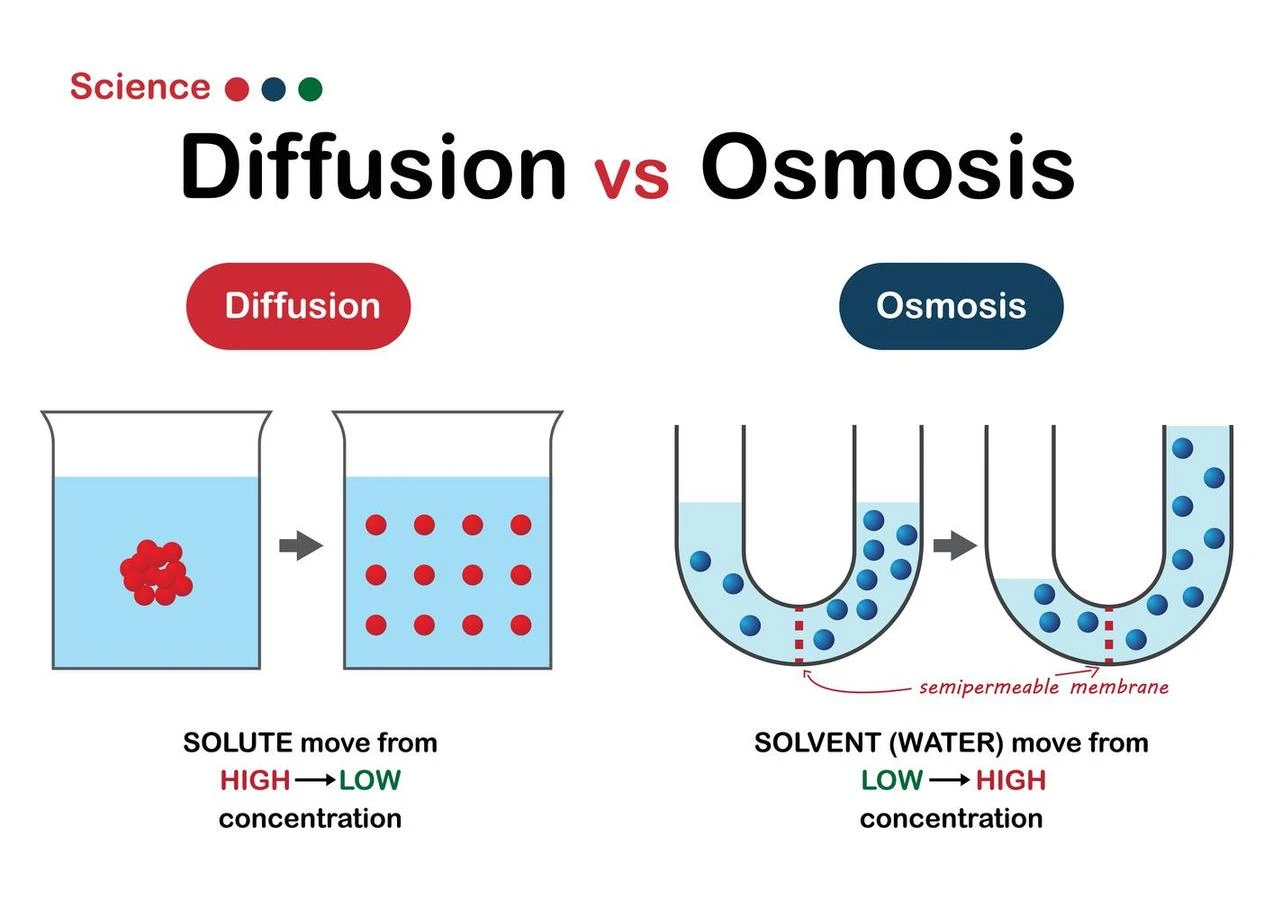
Diffusion
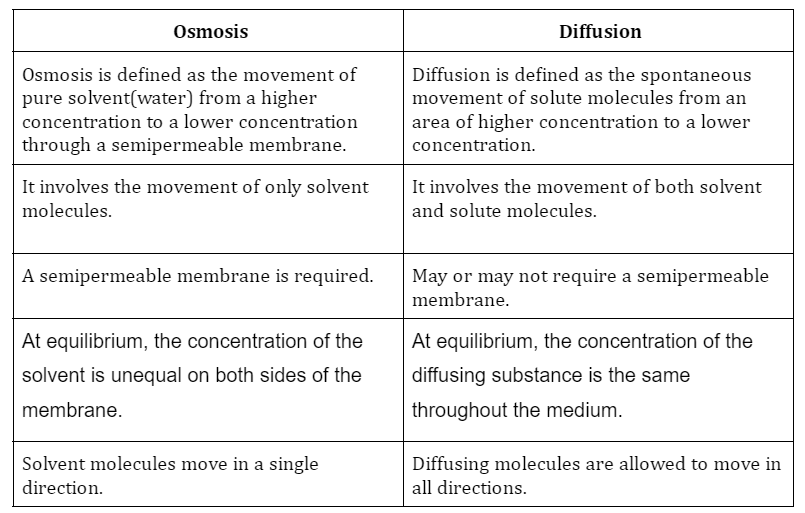
- The spontaneous movement of a molecule down a concentration gradient is called diffusion. For example- diffusion is the spreading of a dye in water.
- It is referred to as a passive process because it doesn’t require any energy input.
- Concentration gradients and the random dynamic movement of the solute molecules are the only factors that influence diffusion.
- Diffusion keeps going until there is equilibrium i.e until there is an equal concentration of the solute on both sides.
- Diffusion happens through a plasma membrane in biological systems.
Types of diffusion
There are two types of diffusions which are explained below
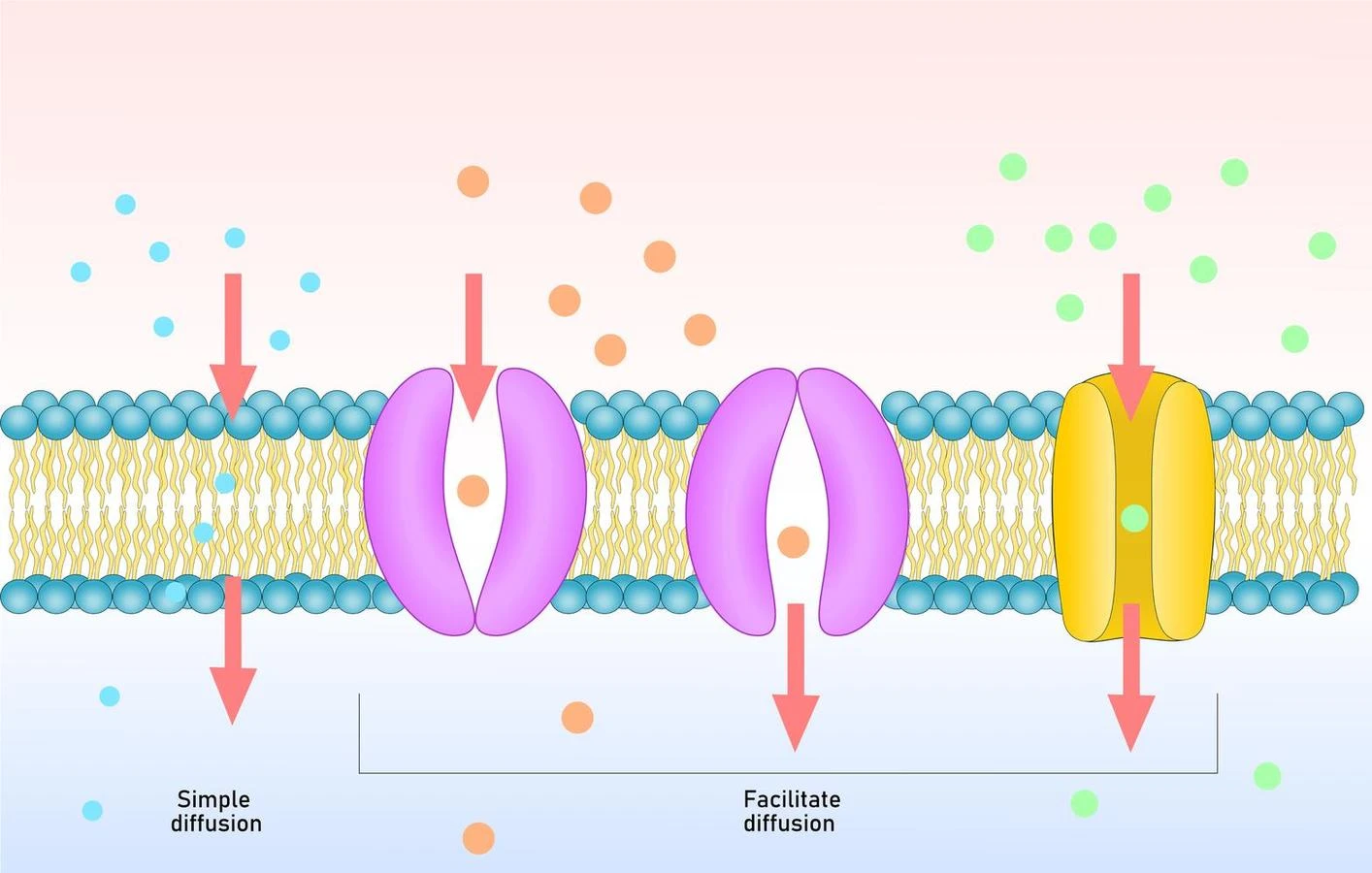
- Simple Diffusion-
- Simple Diffusion is also known as Independent Diffusion.
- This type diiffusion does not require the involvement of membrane proteins.
- The relative concentration of the molecules inside the cell as compared to outside the cell determines the direction of the flow of the molecules.
- Simple diffusion is non-selective and permits the passage of all non-polar molecules across a lipid bilayer.
- Facilitated Diffusion
-
- Some molecules cannot move across the cell membrane due tu their hydrophilicity.
- Thus, the movement of such molecules and charged ions is mediated by certain membrane proteins. This process is termed facilitated diffusion.
- The channel proteins and the carrier proteins are the two different types of membrane transport proteins.
- Polar and charged molecules can cross the cell membrane through the process of facilitated diffusion.
Factors affecting rate of diffusion
- Partition Coefficient-It represents the ratio of a solute’s concentration in two phases when both the phases are in equilibrium with one another. The greater the solubility of a solute, the greater will be its partition coefficient. A substance can pass a biological membrane more readily if its partition coefficient is larger.
- The concentration of the solutes-Fast diffusion occurs when the concentration gradient is higher.
- Temperature- The molecules’ kinetic energy is increased by an increase in temperature, which causes them to travel more quickly across the membrane.
- Mass-Heavier solutes diffuse slowly as compared to lighter solutes.
- The density of the solvent- As solvent density rises, the rate of diffusion declines.
- Nature of the solutes- Lipophilic and non-polar compounds can easily cross the plasma membrane as compared to hydrophilic and polar compounds.
Osmosis
- Osmosis is the process by which solvent molecules move down a concentration gradient and across a semipermeable membrane (i.e., higher to lower concentration).
- It is the net movement of solvent molecules from a high water potential region to a low water potential region.
- Osmosis results in equal solute concentrations on both sides of the membrane.
- The transfer of water from locations with high water potential to those with low water potential is also termed osmosis.
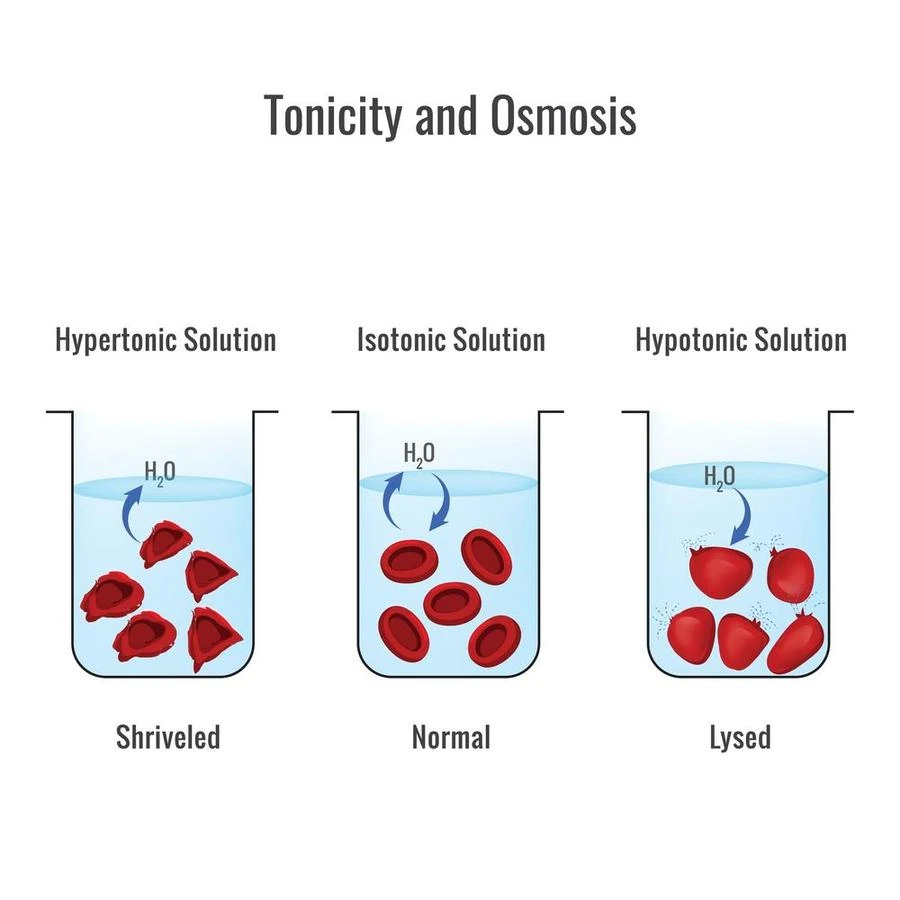
Tonicity
Pertaining to the proportional solute concentrations of a cell and its surroundings there are three different tonicities, they are-
- If a cell is placed in a hypertonic environment, that is, an environment in which the solute is present at a higher concentration than within the cell, the water travels out of the cell and into the surrounding environment. This causes the cell to lose volume and become flaccid. Plasmolysis, a severe condition in which the cell membrane separates from the cell wall and ruptures.
- Water moves into the cell and causes it to swell if the external environment is hypotonic, that is, if the environment has a lower concentration of solute than the cell. Here, the cell gets turgid.
- There is no net change in the volume of the cell if the external environment is isotonic, or if it contains the same number of solutes per litre of solution as the interior of the cell.
Osmolarity
- The number of solute molecules per litre of the solvent is referred to as osmolarity.
- Simply said, osmolarity is a measurement of the amount of solute in the solution.
- As a result, referring to a solution as having low osmolarity suggests that its solute concentration per liter of solution is lower.
- In biology, a cell’s osmolarity is crucial in determining how easily water can move through a bilayer.
- If a semipermeable membrane is used to separate two solutions with differing osmolarities, water from the solution with the lower osmolarity will pass through the membrane and into the solution with the greater osmolarity.
Difference between Osmosis and Diffusion
Summary
- The spontaneous movement of molecules along a concentration gradient is known as diffusion.
- In biological systems, diffusion across the cell may be independent or facilitated by membrane proteins
- Osmosis is the transfer of solvent molecules across a semi-permeable membrane along a concentration gradient.
- Osmosis and diffusion vary primarily in that the former takes place through a semi-permeable membrane while the latter can happen in any medium.
Frequently Asked Questions
1. What is Osmotic Pressure?
Ans: Osmotic pressure is the amount of force required to stop solvent molecules from passing through a semipermeable membrane into a solution. Temperature and concentration both have an impact on osmotic pressure. Osmotic pressure rises as concentrations and temperatures rise.
2. What are channel proteins?
Ans: A channel protein is a type of transport protein that functions as a pore in the cell membrane to allow water molecules or small ions to pass through. Aquaporins, which are water channel proteins, enable rapid water diffusion across the membrane. Ions can diffuse across the membrane through ion channel proteins.
3. Give two examples of osmosis and diffusion each.
Ans: Red blood cells’ enlargement when exposed to freshwater, and absorption of water by plant root hairs are examples of osmosis
The fragrance of perfume spreading throughout a room and the passage of tiny molecules across a cell membrane are both examples of diffusion.