Introduction
In the periodic table, all elements are ordered by following their atomic numbers and periodic characteristics. Antoine Lavoisier tried to classify elements as metals or non-metals in 1789. Johann Wolfgang Dobereiner, a German physicist, attempted to assemble atoms in atomic mass-increasing triads later, around 1817, but this method was unsuccessful for all elements. The elements were later connected depending on the atomic masses by scientist John Newland, who was also unsuccessful. After numerous experts, Dimitri Mendeleev, a Russian chemist, begins to classify elements according to their atomic weights and other physical and chemical characteristics. His periodic chart contained 63 elements in all. Later, his hypothesis was unable to organize every element. Henry Moseley, a scientist, revised the law of the periodic table in 1913 and organized the elements depending on their atomic numbers.
Dobereiner’s law of triads
The “Triad” is a set of three elements that Dobereiner developed by grouping together elements with comparable qualities. By following their atomic masses, he organized three elements so that the mean of these elements’ atomic masses is the same as the mass of the middle elements. For instance, he placed elements with atomic weights of 6.9, 23, and 39 together, including lithium (Li), sodium (Na), and potassium (K). Their average atomic mass, which is 23, is the same as that of sodium. However, he only could put together three of these groups using the known elements, for which his theory of triads was inaccurate.
Newland’s law of octaves
On his table, he arranged about 56 recognized elements. He discovered that each eighth element had a property in common with the first element. He, therefore, equated the elements to musical octaves. Newland’s law of octaves is the name given to his hypothesis because of this.
The eighth element after sodium is potassium, which shares several characteristics with sodium. As a result, they are organized in a single column. He placed several elements in the same category like nickel and cobalt, as well as chlorine and bromine, which do not match any qualities with nickel and cobalt.
Mendeleev’s periodic law
The atomic mass influences the properties, according to Mendeleev’s periodic law. This suggests that when the elements are organized in the sequence of their atomic weights, the physical and chemical characteristics of the elements repeat periodically. The periodic table refers to Mendeleev’s arrangement of the previously known elements in a sequence of increasing atomic masses. Columns are rows, placed vertically that include elements of the same features. Periods was the term used for the rows, placed horizontally.
The modified version of the periodic table
Mendeleev’s periodic law was revised by Henry Moseley in 1913 with the help of atomic numbers of different elements. He claimed that an element’s atomic number has a significant impact on how other elements are organized.
According to him, an element’s characteristics are periodic factors of its atomic number instead of its atomic mass. Moving from one element to the next increases the atomic number of molecules. So, using their rising order of atomic numbers as a guide, he organized the elements.
His hypothesis produced the Modern Periodic table by addressing the flaws in Mendeleev’s Periodic Table. In 7 horizontal and 18 vertical columns, he organized the elements into periods and groups accordingly.
Now that you’ve explored the fascinating history of the periodic table and the remarkable contributions of Dmitri Mendeleev and Henry Moseley, we invite you to deepen your understanding and appreciation of all 118 elements and their symbols, you won’t want to miss this comprehensive guide!
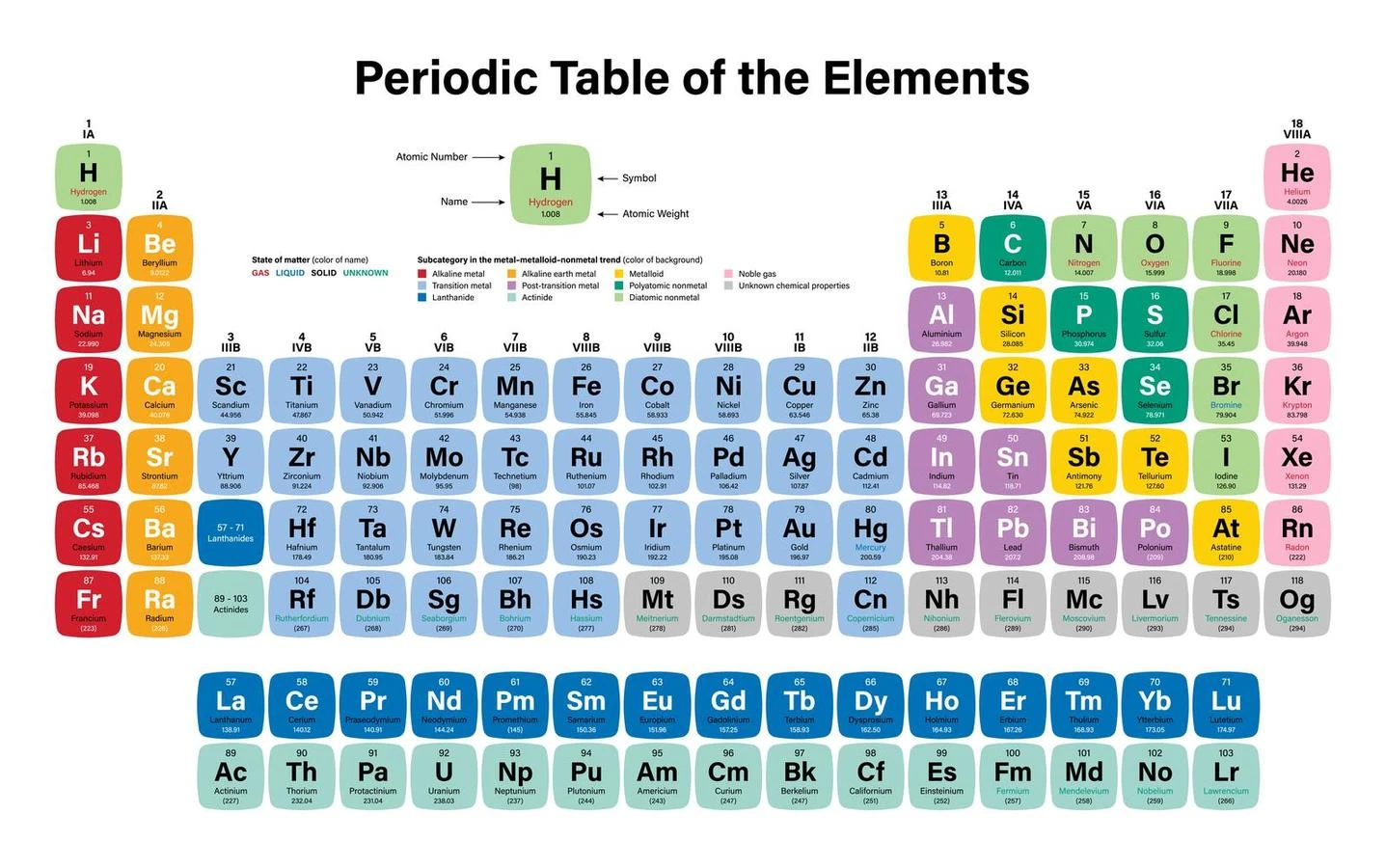
Drawbacks of modern periodic laws
- Doubts remain about the location of hydrogen, specifically whether it belongs to the IA group or the VIIA group. Elements’ isotopes have no location in the modern periodic table.
- Actinides and Lanthanides are not listed in the Current Periodic Table and are preserved discreetly under the table.
- The basis of the law of the periodic table, or the isolation of related elements, was not addressed by the current periodic table. Some unrelated elements have been combined.
Summary
The History and development of the modern periodic table are described here. Numerous scientists like Dobereiner, Newland, and Mendeleev did much research on the periodic table but they faced failure. But later Mendeleev’s periodic table was modified and it went with all the scientific logic. According to this modern periodic table, all the elements are listed in increasing order of their atomic numbers. There are 7 horizontal columns termed as periods and 18 vertical columns depicted as groups. From the modern periodic table, we get an idea about the chemical and physical characteristics of all the elements. Depending on this, the order of size, ionization energy, enthalpy, electron affinity, etc. can be determined properly.
Frequently Asked Questions
1. What element is the least popular?
Ans: Although astatine (At), a member of the halogen family that also comprises fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), may serve as the least common naturally occurring element in the Earth. It is assumed that it exhibits similar features to other Group 17 elements.
2. What does modern periodic law state?
Ans: “The chemical and physical characteristics of the elements are periodic effects of their atomic numbers,” is how the current Periodic law is best represented. The proton number or electron number is the same as their atomic number in the case of neutral atoms.
3. Who designed the modern periodic table?
Ans: To create the current periodic table Neils Bohr, took the idea of the current periodic law that states “the properties of the elements are periodic functions of their atomic numbers,”. It is often referred to as a lengthy periodic table. The current periodic law served as the foundation for its creation. Henry Moseley proposed this periodic law.