Introduction
Water is considered to be the most important material in the daily life of humans. Not only humans but animals and plants also depend on water for survival. Its molecular formula is \({H_2}O\) where two H atoms are covalently bonded with the O atom. The ratio of the number of H and O atoms is 2:1. A major part of the human body is filled up with water. Water is a polar and colorless substance. Due to the polarity of water, any polar substance becomes soluble in water. Looking after the contribution of water on earth, scientists are doing much research on this.
Polarity in water
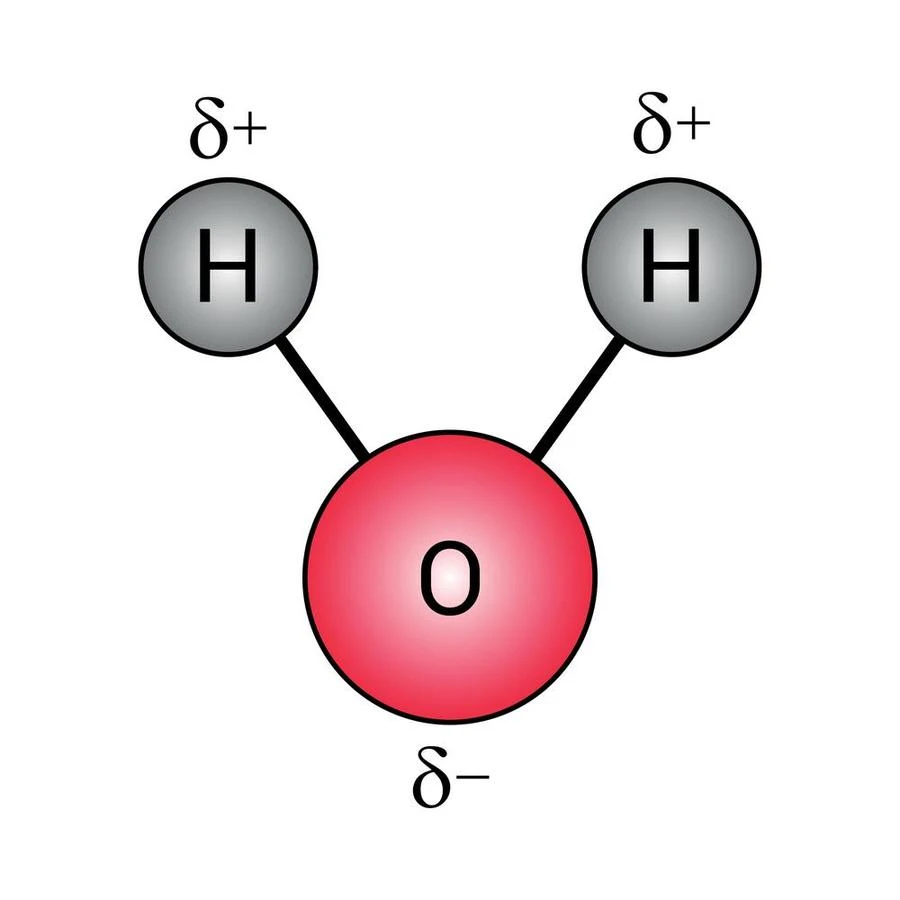
Water can be written by its chemical formula \({H_2}O\). It is composed of two H and one O atom. The electronegativity of O is much higher than H atoms. As a result, A negative charge is generated on the O atom and H atoms become positively charged. Due to this charge separation, water becomes a polar compound.
Water as ‘Universal Solvent’
Water is a polar liquid. Most of the salts contain both positive and negative charges in their structure. As a result, they are polar in nature. water is also a polar liquid. That is why the salts become soluble in water. Not only salt but many other compounds can also easily be dissolved in water. Due to the universal use of water as a solvent, it is called a ‘Universal Solvent’.
Features of water
- Water has very high surface tension and high specific heat.
- It easily contains heat.
- The conducting power of electricity in water is very low. This conductivity increases when the number of ionic substances in water increases.
- Water is a colorless, tasteless and odorless liquid.
Involvement of water in chemical reactions
- Redox reaction: Water behaves as both oxidizing and reducing agent. In the reaction of water with any metal, water is reduced to hydrogen. Hence it can behave as an oxidizing agent. Again when any electronegative element reacts with water, water is oxidized to oxygen. Then water becomes a reducing agent.
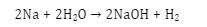
- Reaction with acid and base: Water can react with both the acid and the base. That’s why it is called ‘amphoteric’. The pH of water is always 7 i.e. it is neutral in nature. It can accept protons from acids like H2S and can donate protons to bases like NH3.
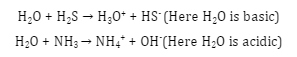
- Self-ionization of water: Small extent of self-ionization occurs in water. Water ionizes to for
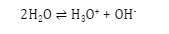
Importance of water in our lives
- Maintain cellular structure: Water can get into various cells and produce pressure so that air can’t be filled in it like a balloon. so the actual cell structure is maintained. Water always helps to maintain the proper molecular form of the cells in plants.
- Daily activities: In our daily life, water helps in various activities like cleaning, washing, bathing, cooking, etc.
- Farming: Farming is totally based on water. With the changes in the amount of rainwater, the growth of crops is hampered a lot.
- Control the pH of the body: Water controls the temperature of our body as well as the pH of our body.
- Enhancing body function: consumption of enough water can enhance the function of the brain. It helps in the easy digestion of foods. Water acts as a lubricant and fills the joints of our body with fluids. It can lower the risk of a massive heart attack in the human body.
Summary
Water is the most vital fluid on the earth. Without water, the survival of humans, animals, and plants will be uncertain. The chemical formula of water is \({H_2}O\). Water is polar in nature. So it can dissolve most of the salts in it. Not only salt, but numerous compounds also become soluble in water. That’s why water is called a “Universal Solvent”. Water can enhance many functions of the human body. About 75 % of the human body is composed of water. Water is amphoteric in nature. For this reason, it is involved in many chemical reactions. Water is also essential for many industrial purposes. It is also used in several activities of our daily life. Every form of life on the earth will be deeply affected without water.
Frequently Asked Questions
1. How will life be without water?
Ans: Rapid dehydration will result in intense thirst, exhaustion, and, eventually, organ malfunction and death. On the first day without water, one can feel mildly lethargic and thirsty, but by the following day, they might be in organ dysfunction. Everyone is not affected by dehydration in the same manner.
2. What are the consequences if we don’t safeguard our water supplies?
Ans: If water is not conserved, a suitable water source may be lost, which could have catastrophic consequences. This included higher costs, less food supply, health hazards, and civil turmoil. It promotes the survival of our ecology.
3. Why is it so crucial to save water?
Ans: Fuel can be saved by conserving water. Reducing daily water intake also lowers your overall carbon footprint because it requires energy to heat, purify, and draw water into our homes. Utilizing less water not only helps save water but also to protect wetland habitats that are home to fish, herons, water small animals, otters, and other species.