Introduction
How does a mobile phone’s battery charge when plugged into its charger, or how does the cell in a TV remote control work? All of these questions have answers in the scientific field of electrochemistry. Electrochemistry is the study of both the use of electricity to conduct non-spontaneous chemical reactions and the production of electricity through chemical reactions. To achieve the goal, cells are used. Cells are components that initiate chemical reactions that produce or generate electricity.
What is an electrochemical reaction?
An electrochemical reaction is any process that is initiated or accompanied by the flow of electrical current, and typically involves the transport of electrons between two substances—one solid and one liquid. An electrochemical reaction occurs when a solid electrode and a material, such as an electrolyte, interact. This flow causes the reaction to release or absorb heat by producing an electric current to pass across the electrodes. When, for example, two electrodes in contact with one another initiate an oxidation and reduction (redox) reaction, the oxidation number of all the atoms involved in the reaction changes.
The process of electrochemical reaction
The properties of the negatively charged\(\;{e^ – }\)determine how matter interacts with an electric current as it flows through a system. Because protons are positively charged matter units found in elements, groups of atoms, or molecules, the electron, the fundamental unit of electricity, is drawn to them. This attraction is comparable to the chemical attraction that particles have for one another. Every chemical reaction changes the structure of an atom’s electrons, and the liberated electrons can either join with matter particles to form reductions or be ejected by them (oxidation).
Faraday’s rules define the quantitative relationship between a free electron in a current flow and the atoms of a substance, where they cause a reaction. Electrochemical process components are also known as ionic conductors or electrolytes.
What is an Electrochemical cell?
An electrochemical cell is a system that can generate electrical energy from spontaneous chemical reactions. The chemical processes that occur during this process are known as redox reactions. During redox reactions, electrons are transferred between chemical species. They are also referred to as galvanic or voltaic cells. An electrochemical cell is illustrated by the Daniell cell.
The following are the essential components of an electrochemical cell:
- An electrolyte is a substance found between electrodes that, when dissolved in polar solvents such as water, produces freely flowing ions, resulting in an electrically conducting solution.
- Electrodes are solid electrical conductors that are used in electrochemical cells and are made of good conductors, such as metals.
- They are available in two varieties:
- The Cathode is the area of the cell where reduction takes place.
- The anode is the part of the cell where oxidation takes place.
- A salt bridge connects the oxidation and reduction halves of an electrochemical cell, completing the circuit. It is brimming with KCl and other saturated salt solutions. The bridge is required for the ions in the solution to flow between half-cells.
What are the different kinds of electrochemical cells?
There are two major kinds:
- Galvanic Cell / Voltaic Cell: Chemical energy is converted to electrical energy in these electrochemical cells.
- Electrolytic Cell: In these cells, electrical energy is converted to chemical energy.
Explain its operation
- Working Principle
The fundamental operating principle of an electrochemical system is the transfer of\(\;{e^ – }\)produced by a redox reaction occurring in it, which results in an electric current.
- Working Mechanism
When the switch is turned on after an electrochemical cell has been fully assembled, the galvanometer of the external circuit deflects. The needle of the galvanometer moves in the direction of the beaker containing the copper sulphate solution. It indicates that the current has changed direction from the copper sulphate solution beaker to the zinc sulphate solution beaker. When the circuit is completed, a change occurs that causes zinc atoms in the zinc electrode to oxidise and Cu atoms in the copper rod to reduce. Zinc releases two electrons, which copper accepts via an external circuit.
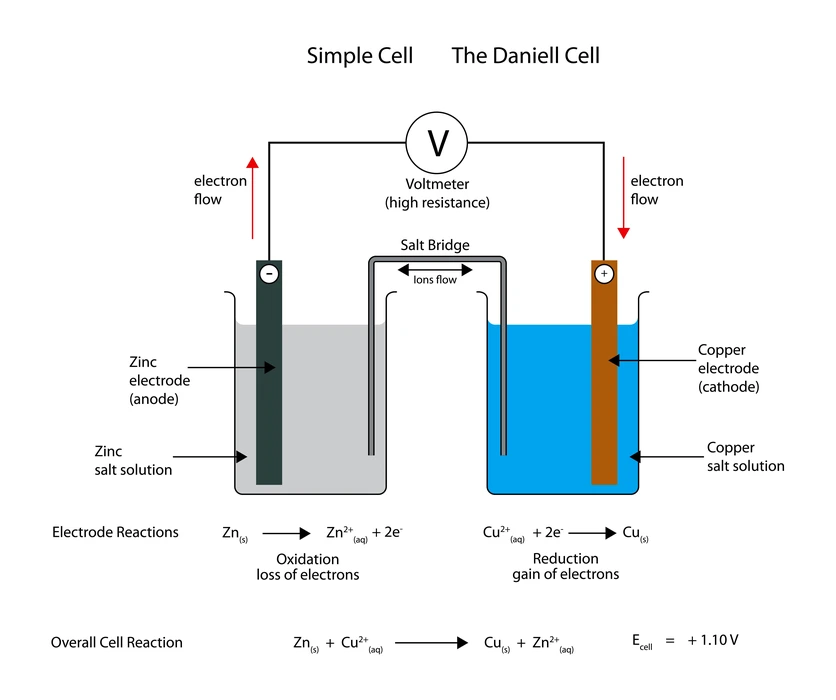
Full redox reaction: \(\;Zn{\rm{ }}\left( s \right){\rm{ }} + {\rm{ }}C{u^{2 + }}\left( {aq} \right){\rm{ }} \to {\rm{ }}Z{n^{2 + }}\left( {aq} \right) + Cu{\rm{ }}\left( s \right)\;\;\;\)
Some applications of Electrochemical Cell
- Many non-ferrous metals are electro-refined in metallurgy using electrolytic cells, yielding very pure metals such as Pb, Zn, Al, and Cu.
- It is used to recover pure Na metal from molten NaCl by storing it in an electrolytic cell.
- Silver oxide batteries are used in hearing aids.
- Thermal batteries are used in Navy gadgets for military applications.
Applications of Electrochemistry
- Electrical batteries are created using the concept of cells. A battery is a device used in science and technology that stores chemical energy and provides electrical access to it.
- Applications in defence (thermal batteries)
- Digital cameras (Li batteries)
- Audio equipment (silver-oxide batteries)
- Electroplating is used for a variety of purposes, including the production of jewellery and the corrosion protection of certain metals.
- Electrochemistry is required in a variety of industries, including the chlor alkali industry.
Summary
Electrochemistry is a fascinating subject. Electrochemical reactions are important to comprehend because they have significant academic and practical implications. Understanding the responses allows us to better understand how everyday objects such as a battery or cell work. Chemical energy can be used to generate electrical energy in electrochemical cells, and electrical energy can be used to generate chemical energy.
Frequently Asked Questions
1. What factors affect electrode potential?
Ans. The reduction potential refers to an electrode’s ability to accept electrons, whereas the oxidation potential refers to an electrode’s tendency to lose electrons. The potential of an electrode is determined by the temperature and metal ion concentration at its surface.
2. Can a zinc pot be used to store copper sulphate solution?
Ans. Copper has a lower reactivity than zinc. As a result, zinc can remove Cu from its salt solution. If the\(\;CuS{O_4}\) solution is kept in a zinc container, copper will be removed from the solution.
\[Zn + CuS{O_4} \to ZnS{O_4} + Cu\]
As a result, the copper sulphate solution cannot be stored in a zinc pot.
3. In the SI system, what is the emf measurement?
Ans. The energy contained in a battery per Coulomb of charge is known as the electromotive force, EMF has a SI unit of volts, which is equal to joules per coulomb.