Introduction
The colloidal solution is one of the significant components of a mixture, along with the two adjacent combinations: true solutions and suspension solutions. In different physical and chemical procedures, all three solutions have variable characteristics and properties, and the significant difference lies in the particle size, appearance, and separation procedure. The three solutions have distinct reactions to the various chemical processes. The dissolving properties of the mixtures differ between the three mixtures due to the variable nature of the solute and solvents involved.
What is a True Solution?
A true solution is a homogeneous combination of two or more substances. In this case, the particle size of the dissolved material in the solvent is less than 10-9 m or 1 nm. Homogeneous means that the mixture’s components form a single phase. The filtration process will not be able to separate the solute from the solution in the solution.
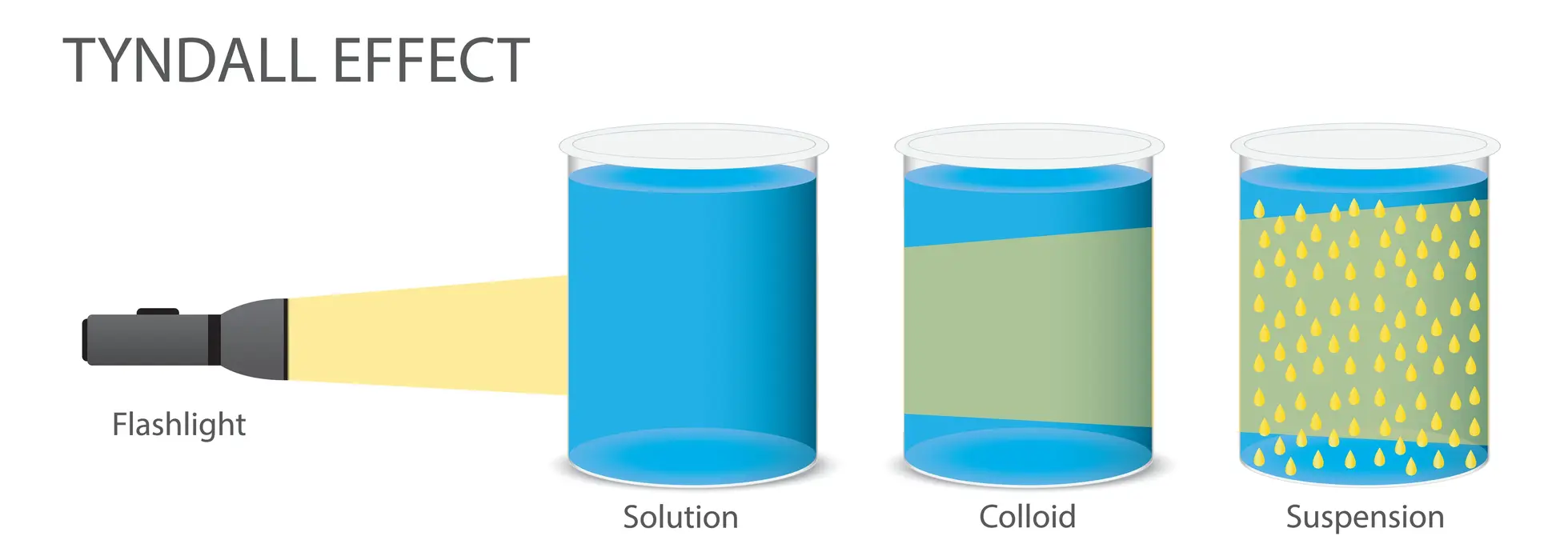
The solute particles do not settle out. The light will never scatter in a true solution. Another distinguishing feature of a genuine solution is its clarity and transparency. A sugar solution in water is an example of a true solution.
What is a Suspension solution?
A suspension solution is a mixture of two or more substances in which the solute particles do not dissolve and remain suspended throughout the solution. Solids are dispersed in liquids in suspension solutions. The particles of the solute are easily visible to the naked eye.
Because the particles are large, they scatter light rays. The path of the ray through the solution is easily visible. Using the filtration method, the particles in the suspension solution can be easily separated. A mixture of chalk and water is a common example of a suspension solution.
An aerosol is a liquid droplet suspension in a gas. Suspensions are further classified based on two factors: a dispersed phase and the dispersion medium.
Want to get an “A” on your Science exams? Let our expert teachers be your guide toward improving your grades and reaching your highest potential. Study Science Subject for classes 6th, 7th, and 8th.
What is a Colloidal Solution?
A colloidal solution is a fluid-suspended mixture of particles of various substances. The particles are microscopically dispersed and soluble/insoluble in this case. Suspension and colloidal solutions are tiny materials that are uniformly distributed. Some of the colloids are translucent due to the Tyndall effect. Some colloids, on the other hand, can be opaque.
You may have heard the term ‘Hydrocolloids’ in the colloids section. This term refers to chemicals that are colloidally dispersed in water. As a result, the solution becomes soluble, altering the rheology of water.
Colloidal systems can exist in three different states: gas, liquid, and solid. Whipped cream and perfume are two examples of colloidal solutions.
Differences between True Solutions, Colloids, and Suspensions
| Attributes | True Solutions | Colloids | Suspensions |
| Meaning | A true solution is a mixture of two or more substances that is homogeneous. | A colloidal solution is a heterogeneous mixture of particles of different substances suspended in fluid that are microscopically dispersed and soluble/insoluble. | A suspension solution is a mixture of two or more substances in which the solute particles do not dissolve and remain suspended throughout the solution. |
| Size | The particles in the true solution are tiny (less than 1 nm) | The particles in the colloidal solution are neither small nor large (1-100 nm). | The particles in the suspension solution are large (more than 100 nm) |
| Visibility to the Naked Eye | The particles are invisible to the naked eye. | The particles are visible to the naked eye. | The particles are visible to the naked eye. |
| Scattering of Light | True solution particles do not scatter light. | The colloidal solution’s particles are large enough to scatter a light beam. | The suspension solution’s particles are large enough to scatter a light beam. |
| Example | Sugar Solution | Blood | Sand in Water |
Summary
So, as you can see, even though these three solutions appear to be the same, they are not. Each of the three solutions has its own set of characteristics. We hope this article answered all of your questions and helped you understand the differences between true solution, colloidal solution, and suspension.
Frequently Asked Questions (FAQs)
1. What is Ultracentrifugation?
Ans. It is the process of using centrifugal force to separate colloidal particles from contaminants. The impure sol is collected in a tube, which is then placed in an ultracentrifuge.
2. Why are the colligative properties of colloids of low order?
Ans. Because colloidal particles are larger aggregates, the particles in colloids are smaller than in a true solution. As a result, when compared to true solution values at similar proportions, measurements of colligative qualities are of low order.
3. Which effect confirms the heterogeneous nature of the colloidal solution?
Ans. The Tyndall effect confirms the colloidal solution’s heterogeneous character. As light travels through a sol, it is scattered by particles, revealing its route and called as Tyndall effect.