Introduction
We currently know about 118 different chemical elements. These elements combine to form numerous compounds. These compounds are classified into Acids, Bases, and Salts based on their chemical properties. “All substances that produce \({H^ + }\) ions when dissolved in water are known as acids, while those that produce \(O{H^ – }\) ions when dissolved in water are known as bases.” When acids and bases are mixed together, they lose their acidic and basic properties, i.e. neutralise, and form salts.
What are Acids
“The word acid comes from the Latin word ‘acidus’ or ‘acere,’ which means sour. The most common feature is their sour taste. In its aqueous solution, an acid produces an ionizable hydronium ion (\({H_3}{O^ + }\)). It causes blue litmus paper to turn red. These dissociate in an aqueous solution to form their constituent ions, as illustrated by the examples below.”
\[HCl{\rm{ }}\left( {aq} \right){\rm{ }} \to {\rm{ }}{H^ + } + {\rm{ }}C{l^ – }\]
\[{H_2}S{O_4}\left( {aq} \right){\rm{ }} \to {\rm{ }}2{H^ + } + {\rm{ }}SO_4^{2 – }\]
\[C{H_3}COOH{\rm{ }}\left( {aq} \right){\rm{ }} \to {\rm{ }}{H^ + } + {\rm{ }}CH3CO{O^ – }\]
What are Bases
Bases are distinguished by their bitter taste and soapy texture. A base is a substance that produces the hydroxyl ion (\(O{H^ – }\)) in an aqueous solution. Bases cause red litmus paper to turn blue.
The bases dissociate in an aqueous solution to form their constituent ions, as shown in the examples below.
\[NaOH{\rm{ }}\left( {aq} \right){\rm{ }} \to {\rm{ }}N{a^ + } + {\rm{ }}O{H^ – }\]
\[Ca{\left( {OH} \right)_2} \to {\rm{ C}}{a^{2 + }} + {\rm{ }}2O{H^ – }\]
What are Salts
When an acidic solution is treated with an alkaline solution or aqueous solution of a metal oxide, a salt is formed, and the solution becomes neutral. A neutralization reaction occurs when \({H^ + }\) ions from an acid combine with \(O{H^ – }\) ions from the base of a metal oxide.
The chemical reactions shown below demonstrate the formation of salt.
\[HCl{\rm{ }} + {\rm{ }}NaOH{\rm{ }} \to {\rm{ }}NaCl{\rm{ }} + {\rm{ }}{H_2}O\]
\[{H_2}S{O_4} + Ca{\left( {OH} \right)_2}\, \to {\rm{ }}CaS{O_4} + {\rm{ }}2{H_2}O\]
Uses of Acids
- Sulphuric acid is used in the production of fertilizers such as ammonium sulphate, detergents, explosives, plastics, dyes, chemicals, etc.
- In the textile, food, and leather industries, hydrochloric acid is used as a dye. It is used to remove oxide films from steel objects prior to galvanization.
- Nitric acid is used in the production of fertilizers like ammonium nitrate, explosives like trinitrotoluene (TNT), plastics, and dye.
Uses of Bases
- Sodium hydroxide is commonly used in the production of soap, as well as synthetic fibre (Rayon) and paper.
- The reaction of calcium hydroxide, also known as slaked lime, produces bleaching powder.
- Magnesium Hydroxide, which acts as an antacid for the body, is used to neutralise excess acid in the stomach and cure indigestion.
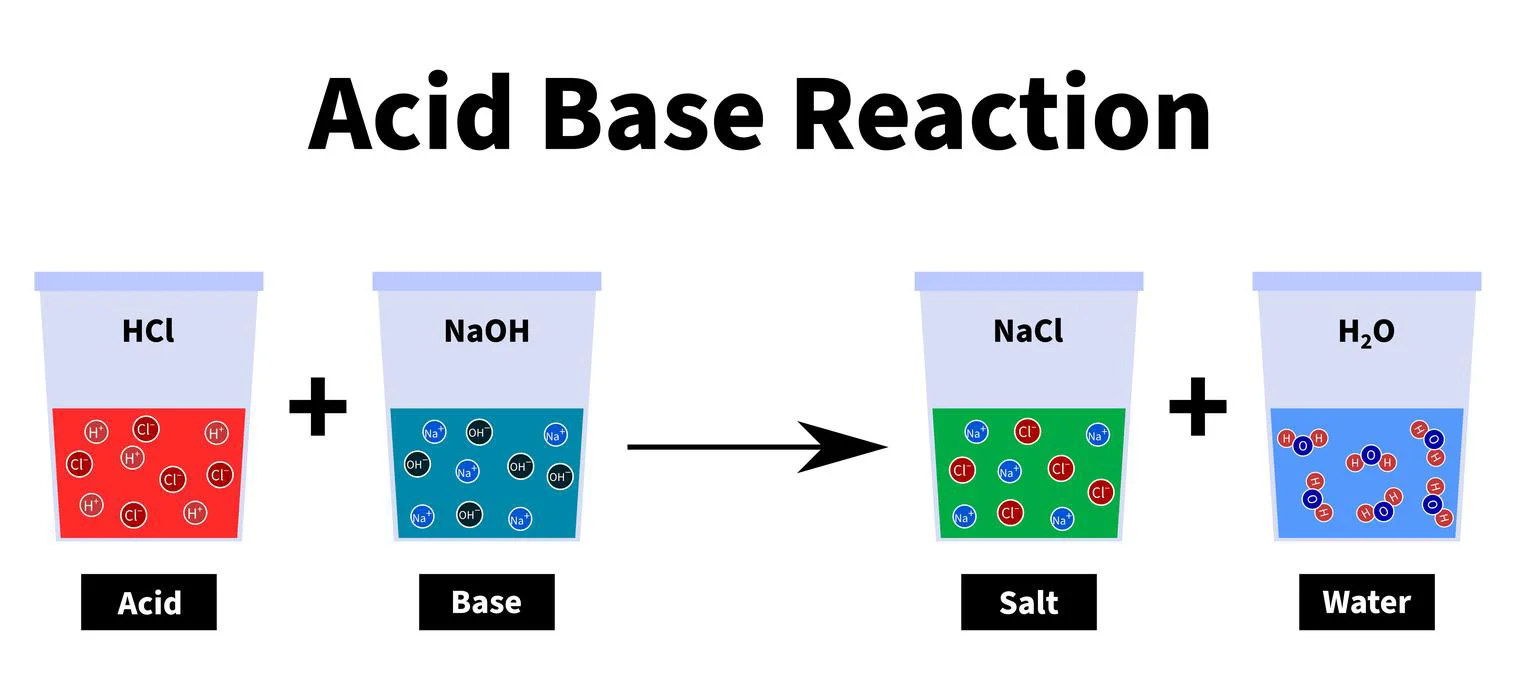
Summary
The compounds are classified into Acids, Bases, and Salts based on their chemical properties. “All substances that produce \({H^ + }\) ions when dissolved in water are known as acids, while those that produce \(O{H^ – }\) ions when dissolved in water are known as bases.” When acids and bases are mixed together, they lose their acidic and basic properties, i.e. neutralise, and form salts.
Frequently Asked Questions
1. What are the main differences between a strong acid and a weak acid?
| Strong Acids | Weak Acids |
| When exposed to water, strong acids completely dissociate into their ions. | In an aqueous solution, weak acids are molecules that partially dissociate into ions. |
| A strong acid solution has a very low pH. | A weak acid solution has a pH of 3-5. |
| It releases all the H+ ions into the solution. | Partially releases all H+ ions to enter the solution. |
2. What are the main differences between a strong base and a weak base?
| Strong Bases | Weak Bases |
| In a solution, a strong base can completely dissociate into its cation and hydroxyl ion. | A weak base partially dissociates into its hydroxyl ion and cation, resulting in an equilibrium state. |
3. Do acids conduct electricity?
Ans. The conductivity is due to the presence of ions. Acids dissociate to form (\({H^ + }\)) ions in solutions. Hence, acids conduct electricity.